
Main content:
- 1.The method of precipitation
- ①Carbonate precipitation
- ②Aluminate precipitation
- ③Boron magnesium, boron lithium coprecipitation method
- 2.Solvent extraction
Lithium has many excellent physical and chemical properties, and its functions and uses are very wide. It is considered as "the energy metal that promotes world progress". Lithium products were initially mainly used in the military, with the rapid development of new energy, metallurgy, aerospace and glass manufacturing industries, people's demand for lithium is climbing year by year, the ubiquity of lithium ion motorcycle batteries prove this point. At the same time, the development of lithium extraction process technology is increasingly paying attention to.
The content of lithium in The earth's crust is only about 0.0065%, a fraction of of it is found in rock deposits, while about 80% of the lithium resources are contained in salt lake brines. China is a major lithium resource country with the second largest total lithium reserves in the world. Among them,salt lake brine resources for 79% of the total lithium reserves in China, mainly distributed in Qinghai, Tibet provinces and so on of China.
Salt lake brine has complex composition and contains a large number of metal and non-metal elements, among which magnesium is the element that most interferes with lithium extraction from salt lake brines. The salt lakes in Qinghai and Tibet of China are mainly of salt lake with high magnesium-lithium ratio.
Among them, the ratio of magnesium to lithium in the salt lake area of Qaidam Basin reach up to more than 50. Because the physical and chemical properties of magnesium and lithium are very close, the separation of magnesium and lithium is very difficult, which becomes the technical bottleneck of large-scale development of lithium resources in salt lake brine. If you want to learn more about specific lithium extraction technology from salt lake brine, please read this article carefully
1.The method of precipitation
The principle of precipitation is to use solar energy to naturally evaporate and concentrate brine from salt lakes, after the removal of boron, calcium, magnesium and impurities, compound precipitator or salting agent was added to the mother liquor to separate lithium in the form of precipitate. The extraction of lithium metal by precipitation method has been used earlier in industry. The process is mature, simple operation and high reliability.
However, the method has poor adaptability to brine with high concentration of alkaline earth metal ions and low concentration of lithium ions. The precipitation method can be divided into carbonate precipitation method, aluminate precipitation method, co-precipitation method between lithium and magnesium, co-precipitation method between lithium and boron and so on according to the different specific technology.
①Carbonate precipitation
Carbonate precipitation method is to vaporize and concentrate the lithium-bearing brine in the salt Lake, and add lime to remove the residual alkaline metal impurities such as calcium and magnesium in the Brine, And then add sodium carbonate precipitator to prepare lithium carbonate products.
②Aluminate precipitation
First, the aluminum-lithium precipitate is obtained by reasonably controlling the aluminum-lithium ratio. Then the precipitate is filtered and calcined at high temperature. Finally, the calcined product is immersed in water to separate aluminum and lithium. After that, calcium, magnesium and other impurities in the lithium solution are removed with precipitant, And after evaporation and concentration, sodium carbonate is added for lithium precipitation reaction to realize the production of lithium carbonate products.

③Boron magnesium, boron lithium coprecipitation method
Boric magnesium coprecipitation method refers to removing magnesium from brine after evaporation, concentration and precipitation of potassium and magnesium mixed salt in salt field, adding alkaline precipitator to control pH value between 8 and 10, and making boron and magnesium co-precipitation under certain temperature and pressure. After solid-liquid separation, NaOH is added to mother liquor for deep magnesium removal, and then soda soda is added to prepare lithium carbonate products. The recovery rate of lithium in this method can reach 80% to 90%.
Lithium boron co-precipitation method refers to the separation of lithium boron and magnesium by adding hydrochloric acid or sulfuric acid and other acidic precipitators to co-precipitate the old halogen of sodium and potassium after impurity removal. The obtained precipitate is washed with water, and then the impurities such as magnesium and calcium are deeply removed. Finally, the precipitator is added to prepare lithium carbonate, and the recovery rate of lithium can reach 75% to 85%.
Boric magnesium, boron, lithium coprecipitation method is suitable for our country magnesia type lithium than lithium salt lake brine production, and this method separation process is simple, high maneuverability, lithium yield is higher, has certain industrial application prospect, but the deficiency is boric magnesium coprecipitation sediment is colloid, solid-liquid separation is difficult, in the process of separation of lithium loss rate of 15% - 20%, Resulting in a huge waste of lithium.
Based on the co-precipitation method of lithium boron, the technological processes of primary freezing, halogenation evaporation, primary evaporation, secondary freezing, secondary evaporation and lithium boron precipitation were used to carry out co-precipitation of lithium boron. The precipitation process developed earlier and has the advantages of mature technology and high operational reliability. However, for the production process of salt lake brine with high MMG/Li ratio in China, there are widespread problems such as large amount of alkaline precipitator, high production cost and poor selectivity to lithium.
2.Solvent extraction
Solvent extraction method is to use the solute in water phase and organic phase solubility or distribution coefficient difference, so that the solute from water phase to the larger solubility of the organic phase, so as to achieve the purpose of solute phase separation, solvent extraction method is one of the main methods of selective separation and extraction of metal ions. At present, the most studied extractants are β -diketone extractant and neutral phosphorous extractant.
①β-diketone extraction agent
β -diketone extractant is also known as chelating extractant. The hydroxyl or carbonyl group on the extractant can form a relatively stable chelating structure with lithium ion, and the corresponding extract can be formed under the action of the extractant, so that lithium can be separated. β -dione extractant has good separation effect in lithium extraction, but β -dione extractant has the problem of high dissolution loss rate under alkaline extraction condition, and the cost of extractant and synergistic extractant is relatively high, so it has not been applied in industry.

②Neutral phosphorus-based extractant
Neutral phosphorus-based extractant mainly include tributyl phosphate, dibutyl phosphonate and trioctyl phosphonate oxide, etc. Under the action of different coextractants or diluents, they can show excellent selective extraction effect for lithium. Qinghai Institute of Salt Lakes used sulfonated kerosene as extraction agent to extract lithium from salt lake brines with a high ratio of magnesium to lithium.
The organic phase after extraction was back-extracted with hydrochloric acid, then the back-extracted solution was treated, and then precipitant was added to prepare lithium carbonate product. Sulfonated kerosene extraction is suitable for lithium extraction from salt lake with high magnesium-lithium ratio, and the separation coefficient of lithium and magnesium is as high as 1.87×105.
This method greatly reduces the production cost, and lithium iron can be separated through one extraction cycle, which simplifies the production process. Although the kerosene extraction system in the treatment of high magnesium lithium ratio salt lake brine shows a good selective separation effect, but the cost is high, the dissolution loss in water is serious, the equipment has strong erosion, and easy to degrade in the process of acid back extraction.
3.Adsorption method
Adsorption method is to use selective adsorbent for lithium ion to bind lithium ion, and then eluting and extraction of lithium ion under the action of eluting agent, so that lithium ion and other impurity ions are separated. The key of this method lies in the selection of lithium ion sorbents. The key to the success of this method is to find lithium ion sorbents with good selectivity, recyclable and relatively low production cost.
According to the nature of adsorbent can be divided into organic ion exchange adsorbent and inorganic ion exchange adsorbent, they mainly rely on the characteristics of the material itself for selective adsorption of lithium ions, because of high selectivity and memory effect, can make lithium and other impurities better separation.
①Aluminum-based adsorbent
Aluminum-based adsorbents are mainly divided into amorphous alumina hydroxide sorbents and aluminum salt sorbents. In the process of lithium adsorption, the larger free acidic hydroxyl group on the surface of amorphous alumina hydroxide adsorbent can promote the formation of hydroxyl-containing complex on the oxide surface and the combination of lithium ions, so that lithium ions can be separated from other impurities.
Aluminum salt sorbents have good selective adsorption performance for lithium ions, but this kind of sorbents mainly exist in powder form, which has the disadvantage of poor fluidity and permeability, and is easy to cause the loss of sorbents in the production process.

②Layered adsorbent
The layered adsorbent is usually a 4 - valent metallic acid salt with a layered structure. In 4-valent metal acid salts, phosphate and ararsenic show high selectivity to lithium ions, and the closer the distance between the sorbents and the diameter of lithium ions, the stronger the affinity of the sorbents to lithium ions, and the superior selectivity to lithium ions.
Thorium ararsenic is an inorganic ion exchanger, whose layer spacing is similar to the size of lithium ions, which can make lithium ions enter the crystal to replace hydrogen, while other ions in brine are blocked outside the crystal, so as to achieve the separation of lithium from other impurities.
③Ion-sieve adsorbent
Ion-sieve adsorbents are highly selective for the extraction and separation of lithium and have become a hot research topic at home and abroad. Under certain conditions, the synthesis of lithium salt precursor, and then lithium ion elution, the metal oxide with pore structure is lithium ion screen adsorbent, the adsorbent has memory effect on lithium ion, can achieve the separation of lithium ion and other impurities ions.
Ionic sieve metal oxides are used to extract and separate lithium, which have the advantages of good stability, strong selectivity and large adsorption capacity, etc., and can be better suitable for lithium extraction from salt lake brine with complex composition. However, the ionic sieve is generally in powder form, and its fluidity and permeability are poor, and the dissolution loss rate is high in the adsorption elution process.

4.Membrane separation process
①Electrodialysis process
Electrodialysis is a membrane separation process with ion exchange membrane as medium and potential difference as driving force. As a mature technology in membrane separation, electrodialysis has been widely used in water treatment. An ion exchange membrane with selectivity for monovalent ions is used for study on concentration of lithium in brine with high ratio of magnesium to lithium. The mother liquor after lithium extraction could be recycled. The research findings show that the electroosmosis process can make the single extraction rate of lithium was more than 80%, and the removal rate of magnesium ion was more than 95%.
The ratio of Magnesium to lithium in brine was greatly reduced, and the concentration of lithium ion was 2-20 g/L after concentration. The key of electrodialysis technology lies in the ion selective exchange membrane, the charged groups on the membrane surface can let the monovalent ions through the membrane hole and prevent the divalent and polyvalent ions from passing through. The mother liquor of lithium extraction can be recycled, and the separation of high magnesium lithium system by membrane technology is bound to become the future research direction. Salt Lake Research Institute of Chinese Academy of Sciences has studied the effect of electrodialysis on the separation of magnesium and lithium from salt lake brine.
In this method, the old brine (Mg/Li weight ratio 1:1 -- 300:1) was concentrated from salt lake brine by sun drying, and lithium was concentrated by circulating, continuous and continuous partial circulating operation processes using univalent selective anion and cation exchange membrane. In this method, the extraction rate of Li+ was higher than 80%, and the removal rate of Mg2+ was higher than 95%, which realized the effective separation of lithium from other ions in brine of salt lake with high mg-Li ratio, and made comprehensive utilization of lithium, potassium, magnesium, boron and other resources in salt lake.
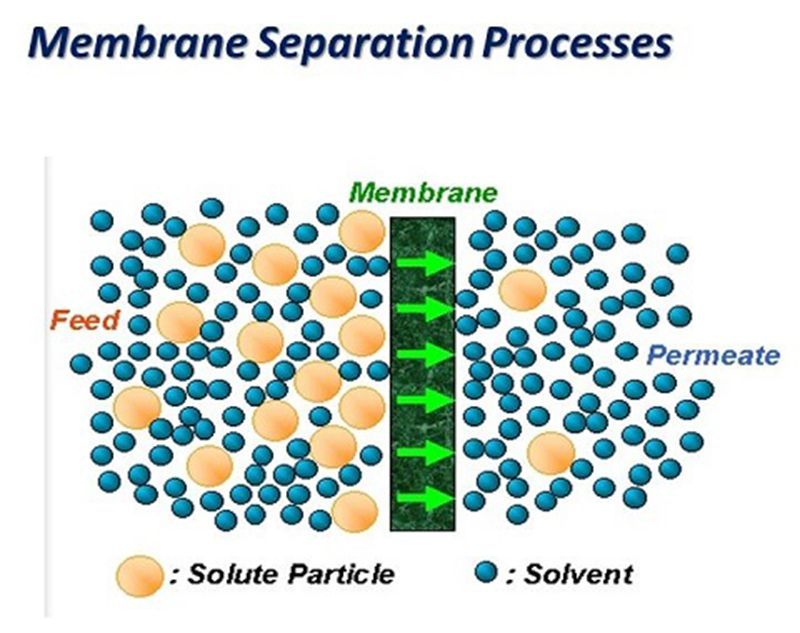
②Nanofiltration membrane
The principle of nanofiltration membrane separation is that the nanofiltration membrane can intercept the metal cations of bivalent and above, and the lithium ion and sodium ion of univalent can pass through, so that the separation method of lithium ion and magnesium ion in the old brine of potassium extraction can be used. A two-stage nanofiltration method was used to separate Mg2 + and Li+ from salt lake brine, and reverse osmosis membrane was used to enrich lithium solution. After the separation operation of nanofiltration membrane, the osmotic water can be used as the influent of reverse osmosis membrane to produce lithium salt, and the concentrated water can be used to extract magnesium salt, so that the inorganic salt resources in salt lake brine can be comprehensively utilized.
5.Calcination
In this method, lithium rich brine after potassium and boron extraction is used as raw material, 50% of the water is removed by sun evaporation to obtain magnesium chloride containing lithium tetrahydrate, and then magnesium oxide containing lithium is obtained through spray drying and high temperature calcination. Then add water to wash, filter, soak lithium, with lime milk to remove calcium, magnesium and other impurities, the solution evaporation concentration to Li+ content of about 2%, finally add soda precipitation, drying to get lithium carbonate products, lithium yield reached about 90%.
Using this method to extract lithium is beneficial to the comprehensive utilization of lithium and magnesium resources, and the consumption of raw materials is less. However, the removal of magnesium tends to make the process more complex, and the equipment corrosion is serious in the production process, and the water evaporation is large.

59% of the lithium resources in the world occur in salt lake brine. Lithium in salt lake brine generally exists in the form of LiCl and Li2CO3, and lithium is generally mixed with alkaline earth metals or alkaline metals, which makes it very difficult to extract and separate lithium. Especially, salt water with high magnesium lithium ratio makes the extraction and separation of lithium more complicated. Therefore, it is of great significance to study the technology of lithium extraction from salt lake brine.
Low magnesium lithium than salt lake lithium extraction technology is relatively mature, precipitation method and salt gradient solar pool method and other methods have been used in industrial application for many years, but the industrial production cost of precipitation method for lithium extraction is high, lithium extraction process produces industrial waste, environmental assessment pressure.
The ratio of low magnesium lithium to salt lake in China is small, and most salt lakes are high magnesium lithium to salt lake, so the industrial application of high magnesium lithium extraction technology is more complex than salt lake. At present, there is no one technology for industrial lithium extraction that can adapt to all salt lake brine types, and lithium extraction technology has a single applicability.
Therefore, comprehensive method can be regarded as a research focus for industrial lithium extraction development in the future. Only by comprehensively applying different lithium extraction technologies, can lithium resources in salt lakes be recovered more efficiently.
If you want to understand the macro lithium resource supplying condition in China, you can read the lithium resource supplying condition in China in our website. By reading that article, you will get to know more about lithium resource supplying condition in detail.
















