|
Main content: |
Other types of secondary batteries, including vanadium-lithium batteries, sodium-ion batteries, magnesium-ion batteries, and lithium-air batteries, are currently researched and applied, and the following will briefly summarize these secondary batteries.
1.Vanadium lithium battery
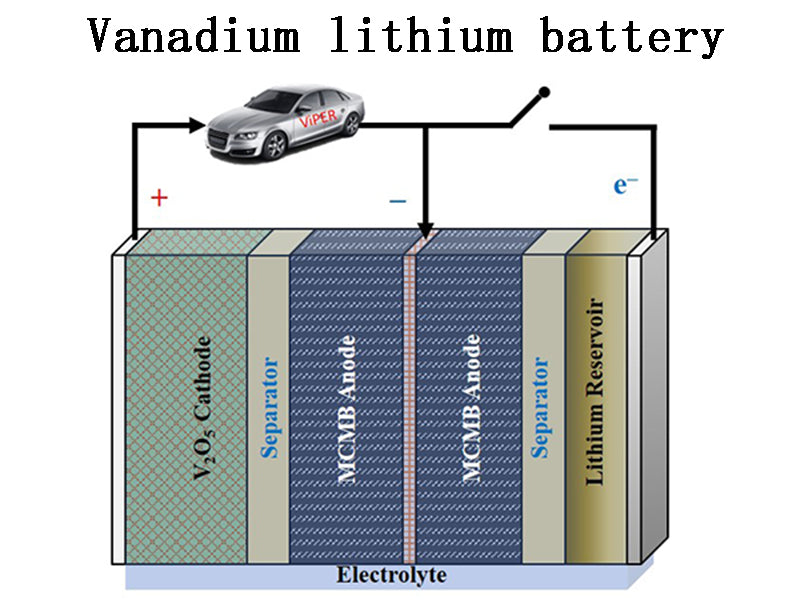
When the secondary lithium battery technology was first developed, technicians generally used lithium metal as the negative electrode material. However, due to technical problems such as lithium dendrites (which are easy to cause short circuits), it was difficult to solve. Currently, lithium alloys are mainly used in lithium ion batteries. Or lithium compounds as negative electrode materials. Despite this, there are still companies and researchers who have adhered to the technological development route of metal lithium as the negative electrode for many years, such as Japan's Fuji Heavy Industries, France's BarCap, etc., which use vanadium oxide as the positive electrode material, and have launched vanadium-lithium batteries for application.
In 2006, Japan's Fuji Heavy Industries released a lithium battery using vanadium pentoxide (V2O5) as a positive electrode material with an energy density of up to 200Wh/kg. The technical principle of the vanadium-lithium battery is as follows: when the battery is made, the lithium metal is electrolyzed, so that lithium ions are doped on the negative electrode. Since there are a lot of lithium ions on the negative electrode side, although V2O5 as the positive electrode material does not contain lithium, it can also be used as an electrode with lithium ions doped on the negative electrode. The technology of vanadium-lithium battery is still under further research and development, and its stability still cannot meet the requirements. The lithium metal polymer battery (LMP lithium battery) product developed by French Barcap uses metal lithium as the negative electrode material, while the positive electrode material uses a resin composite material composed of vanadium oxide, carbon and polymer, and uses solid polymer as the negative electrode material. electrolyte. The LMP lithium battery developed by RatScap has been applied to the electric vehicle product Lucar ev developed by Bollore. The 30kWh IMP lithium battery pack equipped in the Bluccar ev is laid under the floor of the entire cabin and weighs 300kg. Compared with the Nissan Leaf ev, which can store 24kWh of electricity but weighs 280kg, it has a slightly higher energy density. The battery pack voltage is 200~435V. The battery pack can work normally when the external temperature is -20°C~60°C and the internal temperature is 60°C-80°C. The battery pack's warranty coverage is 200,000 km. The BlueCar EV is additionally equipped with supercapacitors as auxiliary equipment, and it is equipped with a motor with a maximum output of 45kW. By using the hybrid power mode of LMP lithium battery and supercapacitor, the BlueCar EV can reach a top speed of 130km/h and a continuous driving distance of 250km. A full charge takes 6 hours, and a 2-hour fast charge restores 50% of the battery's capacity.
2.Sodium batteries
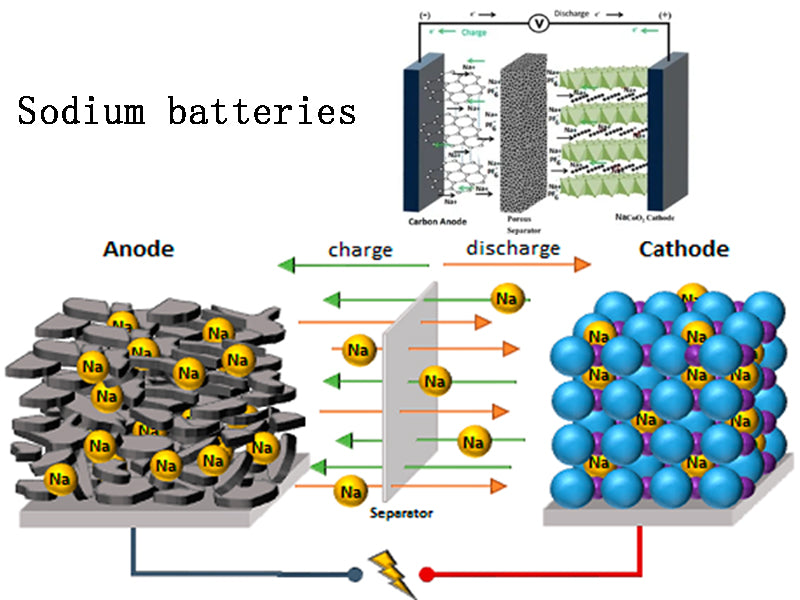
The global reserves of lithium resources are not high. In comparison, sodium is cheap, non-toxic and abundant, so it has received widespread attention. Previous secondary batteries using sodium include the commercial sodium-sulfur battery developed by Japan's NGK company and the sodium-nickel chloride battery developed by Switzerland's MES-DEA company. These batteries use sodium and ceramic solid electrolytes and require an operating temperature of at least 300°C.
1) Sodium Nickel Chloride battery
The sodium nickel chloride (Na-NiCI2) battery was invented by Dr. J Coetger of Zebra power systems in South Africa, so it is called Zebra battery. Germany's AEG Zebra Battery Marketing Gmbh is the main company that studies and trial-produces Zebra batteries. It has carried out trial production of 1-3 generations of battery products, and has been tested and used in cars, trucks and buses. Tests have proved that Zebra batteries have excellent It can meet the requirements of electric vehicle power performance and driving range.
The specific energy of sodium-nickel chloride battery is high, which can theoretically exceed 700Wh/kg. At present, the specific energy of the actual product has reached 100Wh/kg. Therefore, it has great potential for development. The open circuit voltage of sodium-nickel chloride batteries is higher, up to 2.58V. The improved sodium-nickel chloride battery has a higher peak power, which is beneficial to improve the power of electric vehicles. In addition, the sodium-nickel chloride battery also has a high energy conversion rate, no self-discharge, resistance to overcharge and overdischarge, and the battery has Wide operating temperature range and good stability, the cycle life can reach more than 1000 times of charge and discharge. The sodium-nickel-chloride battery adopts a fully sealed structure, which will not burn or explode when subjected to vibration and shock, and is easy to maintain.
In the experimental demonstration area established by Germany on the island of Regen, Mercedes-Benz's 190-type electric car and A-class electric car, BMW's 3-series electric car, and Volkswagen's (VW) Golf electric car , Kadett's electric cars of Opel Company and buses of MAN Company are equipped with zebra power battery as a hybrid battery pack, which effectively increases the mileage of the vehicle. Zbra batteries can be maintenance-free for five years, and some of the experimental vehicles have a range of more than 10,000km. Tests show that Zebra sodium-nickel-chloride battery is a very promising new type of high-energy battery for electric vehicles.
2) Na-ion batteries.
Sodium ion batteries have also received extensive attention from researchers. Although the characteristics of sodium ion batteries are similar to those of lithium ion batteries, the radius of sodium ions is at least 35% larger than that of lithium ions. The limitation of physical size makes sodium ions intercalate and desorb at the positive and negative electrodes. The reaction rate is lower, and the stability of the cathode material for sodium-ion batteries is also higher. The latest research progress of sodium-ion batteries is shown in Figure 1.
The cathode materials of sodium-ion batteries include oxide type and polyanion type; the anode materials include carbon-based materials, titanium-based materials and alloy materials; the electrolytes mainly include organic solvent electrolytes and gel polymer electrolytes. Although various materials have their own advantages and limitations, many researchers believe that the sodium secondary battery system may gradually replace the lithium ion battery. The main problem of the current sodium ion battery research is how to match the different material systems. Cheap and high-performance materials are the key to the commercialization of sodium-ion batteries. While learning from the development experience of lithium-ion batteries, it is also necessary to understand the major differences between the two. A large number of research results on different types of positive and negative electrode materials and structural systems show that the continued in-depth exploration of sodium salt compounds is the key to sodium-ion batteries. important path for rapid development.
3.Magnesium-ion battery
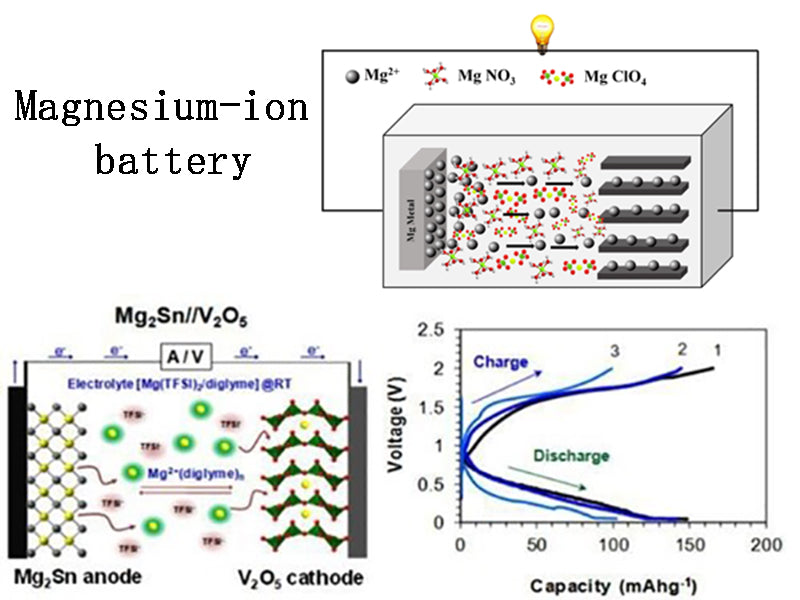
Magnesium is an active light metal with many similarities to lithium in its chemical properties and ionic radius. Compared with lithium, the electrode potential of magnesium-ion batteries is higher (-3.03V for lithium-ion batteries, -2.37V/acidic, -2.69V/alkaline for magnesium-ion batteries), and the theoretical specific capacity is relatively low (lithium-ion batteries are -2.37V/acidic, -2.69V/alkaline). However, magnesium materials have the advantages of low price, low environmental pollution, high melting point (649 ° C), easy processing, and good safety. Most of the materials that can be embedded in the cathode of magnesium-ion batteries are inorganic transition metal compounds, including oxides, sulfides, borides, polyanionic compounds, and sulfur-containing conductive materials. There are two main factors restricting the development of magnesium-ion batteries:
①The solvation of magnesium ions is strong, and there are few matrix materials for magnesium ions to be embedded;
② Even in an aprotic solvent, a dense passivation film is easily formed on the surface of the magnesium electrode, which makes the electrochemical reaction on the surface of the magnesium electrode relatively complicated. It is necessary to find an alternative electrolyte system to solve the problem of the passivation film. In a word, how to solve the electrochemical dissolution and deposition balance of magnesium in the electrolyte and improve the working voltage and energy density of the battery is an urgent problem to be solved at present.
An ideal cathode material for magnesium-ion batteries should have the following characteristics:
① has high specific energy;
② has a high electrode potential;
③It has good reversibility of charge-discharge reaction; it has good chemical stability in electrolyte and low solubility
⑤ has high electronic conductivity and so on.
Pelion Technologies of the United States is actively developing magnesium secondary batteries with high energy density. The company uses computer technology to analyze and predict material properties to accelerate the process of material synthesis and electrolyte optimization, thereby identifying new magnesium anode materials with high energy density and compatible electrolyte materials. According to a statement from the U.S. Department of Energy's Advanced Research Projects Agency, Pelion Technologies is expected to break through current battery technology for electric and hybrid vehicles.
4.Lithium-air battery
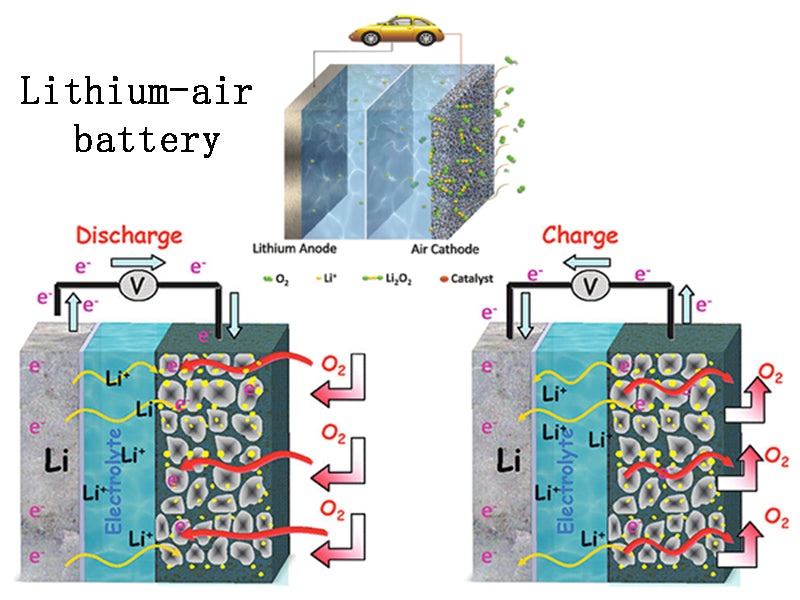
Lithium-air battery is a battery with metal lithium as the negative electrode and oxygen in the air as the positive electrode reactant. During the discharge process, the metal lithium of the negative electrode releases electrons to form lithium ions (Li+), and Li+ passes through the electrolyte material to combine with oxygen and electrons flowing from the external circuit at the positive electrode to form lithium oxide (Li2O) or lithium peroxide (Li2O2) , and remain in the porous carbon substrate of the positive electrode. The open circuit voltage of the lithium-air battery cell is 2.91V.
The lithium-air battery has a high energy density, and its specific energy can reach 5210Wh/kg (with oxygen mass), or 11140Wh/kg (without oxygen mass). It needs to be stored in the positive electrode. Theoretically, since there is no material limit for oxygen as a positive reactant, the capacity of the lithium-air battery depends only on the lithium metal electrode, and its isothermal value is 40.1M/kg, which is similar to the gasoline's 44MU/kg. Therefore, Li-air batteries are very attractive from the point of view of specific energy.
In 1996, KMAbrahan and his colleagues demonstrated for the first time a practical non-aqueous lithium-air battery. This battery uses metallic lithium as the negative electrode, porous carbon as the positive electrode substrate material, and a gel-like electrolyte film between the positive and negative electrodes as the battery. Separators and media for ion transport, electrolyte materials include gel-like electrolytes such as polyacrylonitrile (PAN) and polyvinylidene fluoride (PVDF), and materials such as organic solvents, dry organic polymers, and inorganic metal electrolytes can also be used. Oxygen from the environment enters the cathode region through the pores of the porous carbon and acts as the cathode active material. During the discharge process of the lithium-air battery, the oxygen in the carbon-based pores is consumed, and the generated discharge products are subsequently filled in the carbon-based pores.
Since the cathode of a lithium-air battery is constructed in the same way as a fuel cell, the electrode needs to have the ability to use a catalyst to react with oxygen. At the same time, as a secondary rechargeable battery, it must also have the function of reducing reaction products such as Li2O2, which needs to be further solved in practical application. The Japan Institute of Industrial Technology developed a new type of large-capacity lithium-air battery in 2009. The negative electrode material of the battery is metal lithium, which is immersed in an organic solvent electrolyte containing lithium salts, and the positive electrode uses an aqueous electrolyte, which has a similar structure. For fuel cells, the oxygen in the air reacts with the water-based electrolyte of the positive electrode under the action of the catalyst in the porous carbon material to form an alkaline solution at the positive electrode, which completely avoids the formation of lithium oxide at the positive electrode of the original lithium-air battery. The problem of plugging porous carbon. The separator of the battery uses lithium zinc germanate (LISICON) wafer as the solid electrolyte to prevent the two electrolytes from mixing, and the open circuit voltage of the battery is about 3V. Experiments show that the battery has a very high specific capacity, the value is as high as 50000mAh/g, and the highest is 80000mAh/g. In 2010, Toyota Motor Corporation of Japan released the trial production results of a lithium-air battery with a positive electrode composed of ketjen black, electrolytic manganese dioxide and fluororesin powder (PTFE), and a negative electrode composed of metal lithium materials.
Research and development in lithium-air batteries in the United States is also continuing. For example, IBM has rich experience in material science, nanotechnology, chemistry and supercomputer, so it has obvious advantages in the development of lithium-air batteries. The company plans to use nano-diaphragm technology to develop a water purification system to combine oxygen in the air with water material isolated. Using experience with nanostructures can also distribute the oxygen in the battery to each cell to prevent clogging. Supercomputers can conduct modeling research that effectively guarantees that individual atoms can pass through the nanomembranes in batteries. A technology consortium led by IBM hopes to manufacture lithium-ion power batteries that combine lithium and oxygen elements, and double the performance of current lithium batteries, which can drive electric vehicles for 500 to 800 kilometers on a single charge.
Although many countries and companies have made progress and produced samples in the field of lithium-air batteries, so far, the development of lithium-air batteries is still very difficult. The urgent problems to be solved in this battery technology mainly include how to prevent the two Chronic penetration of electrolyte outside the diaphragm; how to increase the usable temperature range of organic electrolyte, improvement of catalysts, safety issues of lithium materials, and how to recycle unused lithium metal and lithium hydroxide, etc.
5.Zinc-air battery
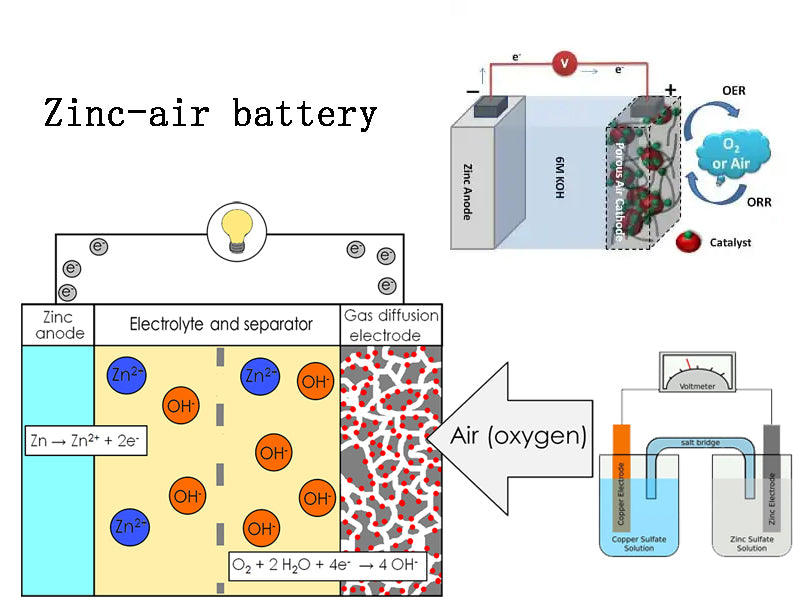
Zinc-air batteries have a history of more than one hundred years since their invention, but it was not until 1995 that Israel Electric Fuel (Electric Fuel) Co., Ltd. first used zinc-air batteries for electric vehicles, which brought air batteries into the practical stage. Dreisback in the United States Electromotive companies, as well as Germany, France, Sweden, the Netherlands, Finland, Spain, Portugal and South Africa and other countries are also actively promoting the application of zinc-air batteries in electric vehicles.
Zinc-air batteries have the following advantages:
(1) The specific energy is larger.
The theoretical specific energy of zinc-air battery can reach 1350Wh/kg. Although the actual specific energy of zinc-air battery can only reach 180~230Wh/kg at present, it is still 4.35~5.5 times that of lead-acid battery. Therefore, it can be expected that after the adoption of zinc-air batteries, electric vehicles will be able to significantly increase the driving range.
(2) Stable performance.
The zinc-air batteries used in groups have good consistency, and there is no unevenness between the individual cells that occurs when other types of battery groups are charged and discharged. The zinc-air battery allows deep discharge, the capacity of the battery is not affected by the discharge intensity and temperature, and can work normally within the temperature range of -20°C to 80°C.
3) Good security.
Zinc-air batteries can effectively prevent fire or explosion caused by leakage and short circuit. In addition, zinc has no corrosive effect, causing no harm and danger to the human body. It can be completely sealed and maintenance-free, which is easy to manage.
(4) The source of zinc is rich and the production cost is low.
Zinc electrodes are also easy to recycle, and the cost of recycling is low, and a waste battery recycling plant can be established. Zinc will not pollute the environment during the recycling process.
6.Aluminum-air battery
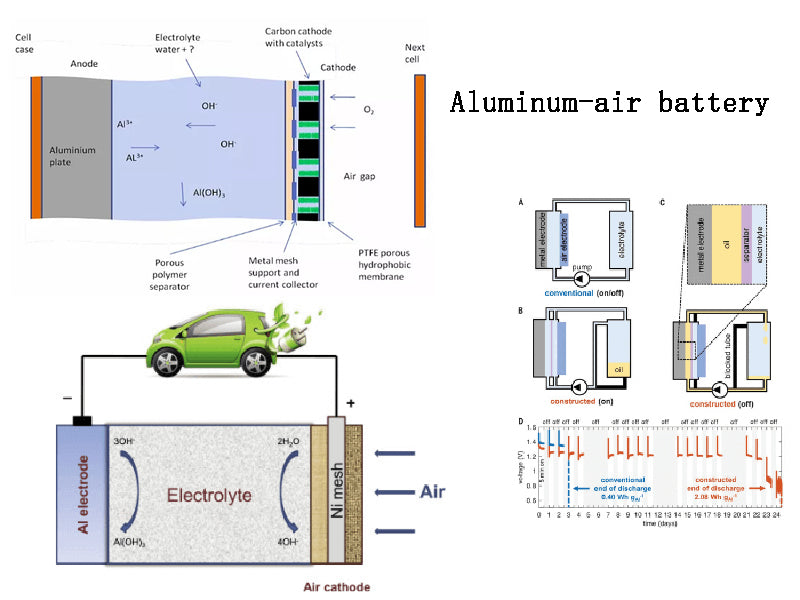
The aluminum-air battery uses high-purity aluminum (with A content of 99.99%) as the positive electrode, oxygen in the air as the negative electrode, and potassium hydroxide and sodium hydroxide aqueous solution as the electrolyte. The chemical reaction inside the aluminum-air battery is similar to that of the zinc-air battery. When the battery is discharged, the aluminum chemically reacts with the oxygen in the air and is converted into aluminum oxide. The development of aluminum-air batteries has progressed very rapidly, and good application results have been achieved in electric vehicles. It is a kind of air battery with great development potential.
Aluminum-air batteries have the following advantages.
(1) The specific energy is larger.
The theoretical specific energy of the aluminum-air battery can reach 8100Wh/kg. Although the actual specific energy of the aluminum-air battery is only 350Wh/kg at present, it is also 7~8 times that of the lead-acid battery, 5.8 times that of the nickel-metal hydride battery, and 5.8 times that of the lithium-ion battery. 2.3 times. Electric vehicles can significantly improve the driving range after using aluminum-air batteries. According to relevant data, an electric vehicle using an aluminum-air battery has set a record of only replacing the aluminum electrode once and driving a mileage of 1,600km in California, USA.
(2) Light weight.
The total energy of the traction power lead-acid battery developed and developed in China is 13.5kWh and the total mass is 375kg, while the total mass of the aluminum-air battery with the same energy is only 45kg, which is only 12% of the mass of the lead-acid battery. Since the battery mass is greatly reduced, the curb weight of the electric vehicle is correspondingly reduced, which can improve the loading quality of the electric vehicle or prolong the driving range of the electric vehicle.
(3) Aluminum is not toxic and dangerous, and will not cause harm to the human body.
In addition, aluminum electrodes can also be recycled and used without polluting the environment.
(4) Low cost.
Aluminum resources are abundant, and large-scale aluminum smelting plants have been established, and the production cost is relatively low. The recycling and reuse of aluminum is convenient, and the cost of recycling and regeneration is also low.
Although the aluminum-air battery contains high specific energy, it also has disadvantages such as low specific power, slow charging and discharging speed, voltage lag, and high self-discharge rate. Effective solutions need to be taken in the application, such as using The thermal management system is used to prevent the overheating problem of the aluminum-air battery when it is working, and the problem of slow charging of the aluminum-air battery can be solved by replacing the aluminum electrode.
















