Main content:
Lithium battery has been identified by the United States Advanced Battery Council (USABC) as a technology that requires long-term research. Different application scenarios use different lithium batteries. In some applications, lithium polymer batteries have replaced lithium ion batteries. Although their specific energy is slightly lower, they are safer. Of course, safety issues (fires) caused by overcharge, overdischarge, and short circuits are important factors that require constant attention for lithium battery until a new lithium battery technology is put into commercial use.
Among lithium battery, metal lithium battery is technically very different from lithium ion batteries. The negative electrode of a lithium-metal battery consists of metallic lithium, a material prone to serious safety problems; lithium-ion batteries keep the lithium in an ionic state due to the addition of an intercalation compound (usually graphite) to the negative and positive electrodes.
1.Metal lithium battery
During the discharge phase of the lithium battery, the anode of the metal lithium battery (that is, the negative electrode composed of metal lithium) undergoes an oxidation reaction. Lithium ions flow through the electrolyte to the cathode (positive electrode), where they are reduced by mixing (reacting with) specific materials (mainly hydrogen materials, see Figure 8-5), thereby providing electrical energy to an external circuit by releasing electrons. During the charging phase, the lithium ions undergo a reverse reaction and are supplied with electrons by an external circuit.
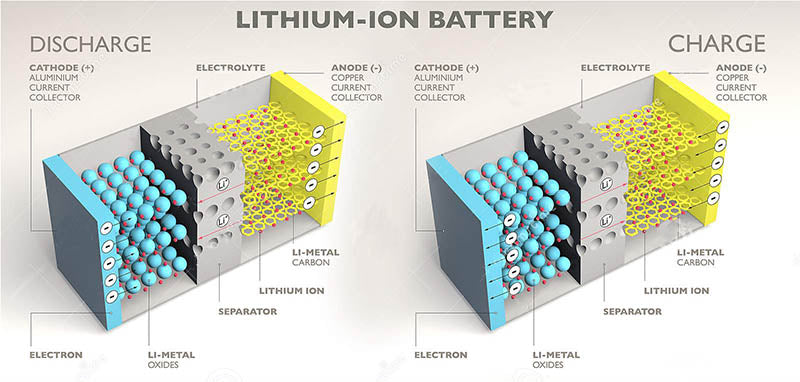
The reaction of lithium-ion batteries on the negative electrode material is different from that of metal lithium battery mentioned above, and the lithium material is replaced by carbon (graphite) that allows lithium to enter freely. The main advantage of the lithium battery with this structure is that the lithium and the electrolyte do not come into direct contact, so the chemical properties of the system can be improved. Compared with pure lithium electrodes, the electrode potential of this lithium battery is slightly larger and the electrode mass is heavier. Therefore, the energy density of this type of lithium battery is reduced from 3828 A·h/kg of pure lithium electrode to 340 A·h/kg of LiC6 compound electrode.
Lithium-ion batteries use two materials so that lithium ions can undergo a reversible reaction. The negative electrode uses a graphite film that has embedded lithium atoms (LiC6), and the positive electrode can use an over-coated lithium oxide material (such as LiCoO2). The liquid electrolyte is usually a carbonate solution containing lithium hexafluorophosphate (LiPF6). The cell voltage of a lithium-ion battery is about 4V, while a typical single cell operating voltage is 3.7V.
The electrolyte of a lithium battery can only be a pure ionic conductor, so it has good electrical insulating properties. In most cases, the electrolyte of this lithium battery is liquid (such as LiAsF6, LiPF6, LiCIO4, etc., dissolved in organic solvents or mixed solvents such as propylene carbonate, ethylene carbonate, dimethoxyethane, etc.). The electrolyte may also be solid, such as an organic compound based on polyethylene oxide or an inorganic compound based on amorphous lithium borate. Polymer electrolytes belong to the family of solid electrolytes, which are composites of polymers (eg, polyethylene glycol PEO) and lithium salts (eg, LiCIO4). For colloidal polymers, lithium salts are dissolved in organic solvents such as ethylene carbonate and cured in polyacrylonitrile (PAN) or PVdF-HFP copolymers. The performance of the solid electrolyte of this lithium battery is generally lower than that of the liquid electrolyte, but it has good ionic conductivity, which can reach the order of 10-5~10-3S/cm.
2.Lithium iron phosphate battery: Lithium battery with very high energy density
Lithium iron phosphate battery is a type of lithium-ion battery. The positive electrode is composed of the active material LiFePO4, while the negative electrode is graphite. The cell voltage of lithium iron phosphate battery is slightly lower (3.2V), but it has better safety, lower cost and better cycle stability.
3.Metal lithium polymer battery
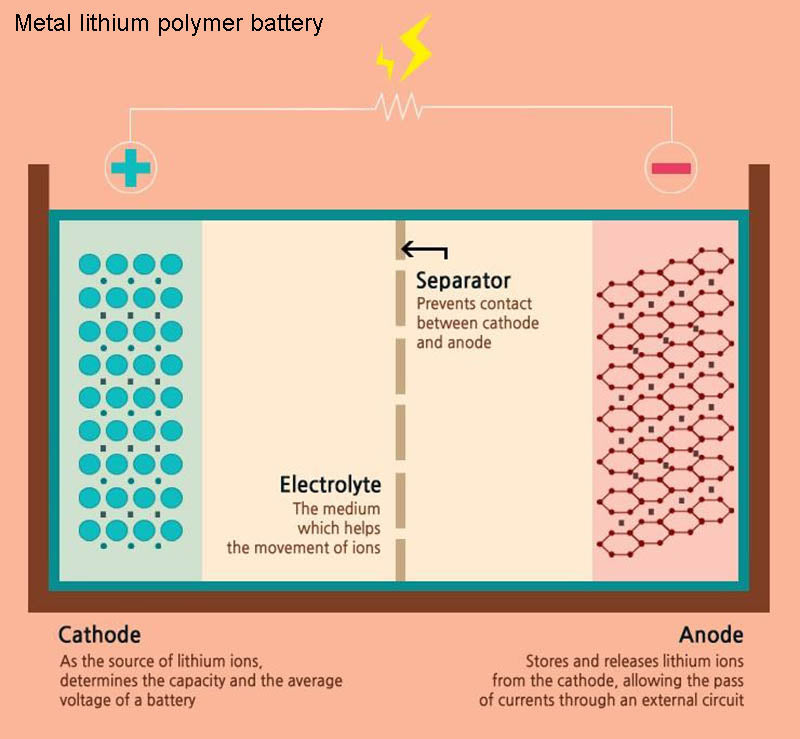
The negative electrode of this lithium battery is metal lithium, the positive electrode is vanadium oxide, and the electrolyte is a polymer based on POE (polyethylene oxide) and lithium salt. The electrolyte of this lithium battery has the best performance at 60~80℃ . This lithium battery is realized by extrusion technology and was developed by French BATSCAP company and Canadian AVESTOR company. Although currently more expensive, this new lithium-ion battery technology will be promising in the future and will eventually be cheaper to manufacture than traditional lithium-ion technology.
The lithium-polymer battery was invented and designed by Professor Michel Armand. It is completely technically feasible, costs $100~150/kW·h, and can operate well at temperatures of 60~150°C without safety concerns. There is some research working on making the battery run at a slightly lower temperature, but this kind of lithium battery won't work at -20°C. However, under suitable temperature conditions it requires very little energy supply. The self-discharge rate of this lithium battery for a week is 10% to 20%. Take the lithium metal polymer battery developed by Batscap as an example. The energy density of this lithium battery can reach 110W·h/kg (110W·h/L), and it works at a temperature of 90°C (internal temperature). ).
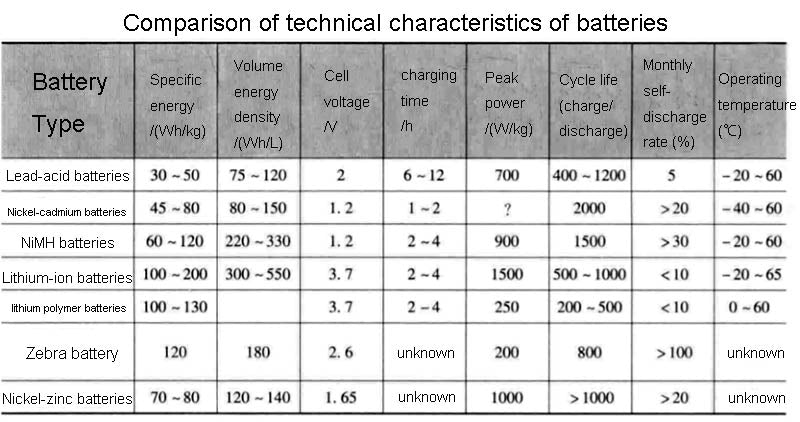
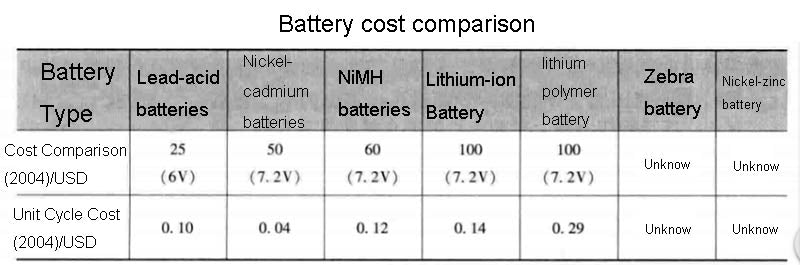
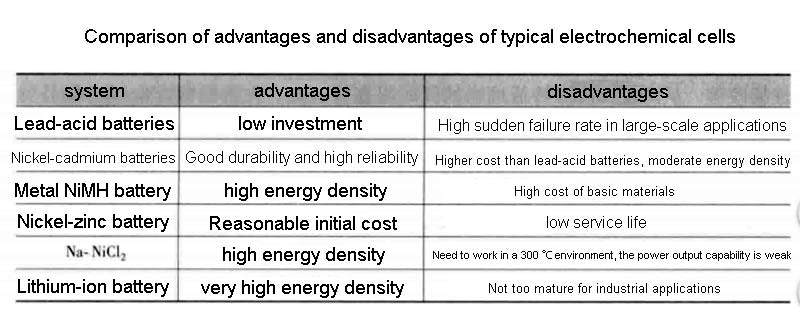
Lithium-ion battery technology would be very attractive if it could eliminate risks in high temperature operating environments. For example, lithium-carbon batteries work well at low temperatures, but due to the use of organic electrolytes, the system heats up when the temperature exceeds 60°C. This kind of lithium battery is not dangerous at small capacity, but the capacity cannot exceed a certain level. A comparison of the technical characteristics, cost, and advantages and disadvantages of some lithium batteries is shown in the figure below.