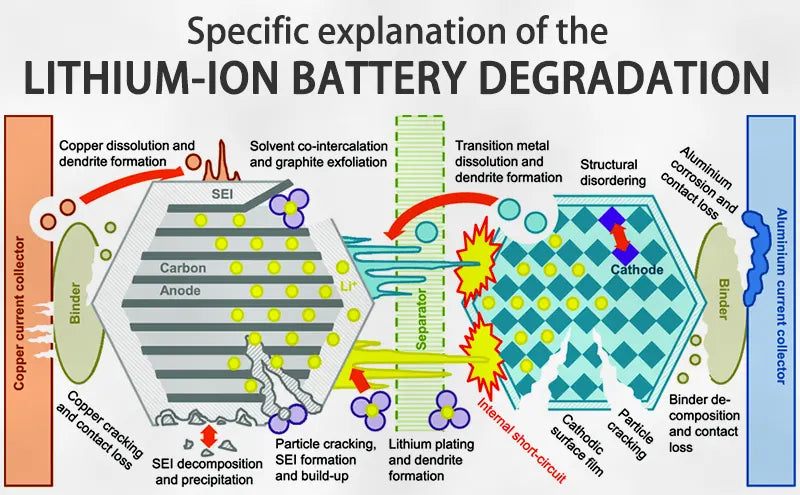
Main content:
On the one hand, lithium ion battery degradation is a natural consequence of use and aging, and on the other hand, it is accelerated by lack of maintenance, harsh use environment, and poor charging operation.
The following will explore the insurmountable problems of lithium ion battery degradation, their causes, and ways to compensate for them.
1. High self discharge rate
Self-discharge exists in various batteries, but improper use can promote the development of this state. The self-discharge rate is asymptotic, and the highest discharge rate appears just after charging, and then gradually decreases.
Nickel-based batteries exhibit a high self-discharge rate. Usually the temperature is higher, and its discharge rate is also increased. The self-discharge rate of nickel-based batteries also increases after hundreds of cycles.
The battery plates begin to expand to squeeze the separator between the electrodes more tightly, forming metal dendritic crystals, causing the lithium ion battery to degenerate.
This is the result of crystal growth (memory effect), which damages the battery separator and increases the self-discharge rate. But the lifepo4 batteries don't have memory effect.
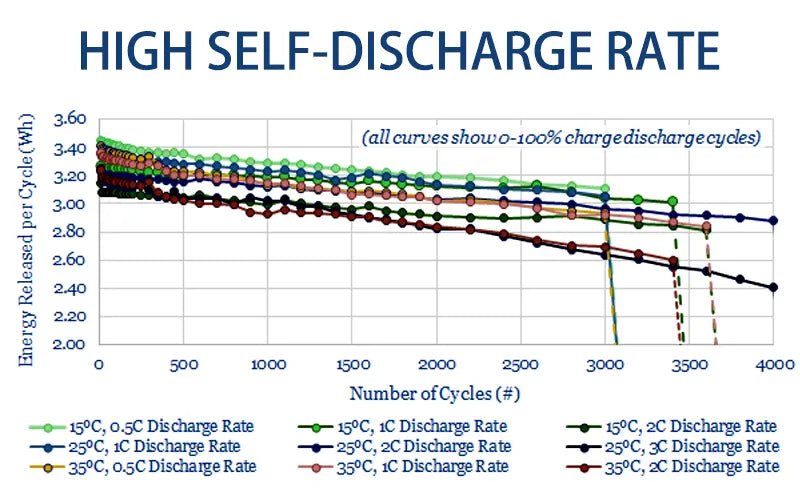
If the nickel-based battery reaches 30% self-discharge within 24 hours, it should be discarded. The high number of cycles and aging have no effect on the self-discharge rate of lithium-based batteries. Repeated deep cycle charging and discharge of lead-acid batteries increases self-discharge.
2. Battery matching
Even with modern manufacturing techniques, it is impossible to accurately predict battery capacity, especially for nickel-based batteries. During the manufacturing process, each battery is tested and classified according to the size of its capacity.
High-capacity class "A" batteries are typically sold as special-purpose batteries at premium prices. Medium-capacity class "B" batteries are used in industrial and commercial products. And low-end "C" batteries are sold cheaply. Cyclic charging and discharging does not improve the capacity of low-end batteries. What you get when you buy a cheap rechargeable battery is a low battery capacity.
● Impact on the lithium ion battery degradation
In battery packs with a large number of batteries, as well as battery packs that need to output large load currents and operate at low temperatures, tighter battery tolerance control is required.
If there is a slight mismatch between the cells in a new battery pack, after several charging cycles, they will be able to balance and adapt to each other. Whether the batteries can be well balanced and adapted is related to whether the battery pack has a long service life and the lithium ion battery degradation.
● The importance of battery matching
High-quality batteries have a more consistent and balanced capacity than low-quality batteries. High-quality batteries should be used for high-end high-power tools, because they can have high durability in large loads and extreme temperature environments.
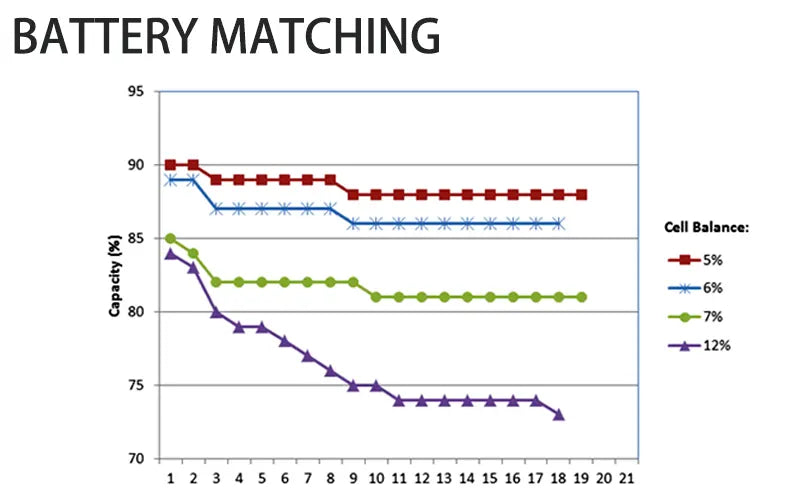
Despite the high cost, the reward is that the battery pack has a longer life and the lithium-ion battery degrades more slowly. When batteries come off the production line, their intrinsic performance matches well. It is important that individual cells within the battery pack meet tight tolerances.
All cells in the pack must reach full charge within the same amount of time and reach the same threshold voltage at the end of discharge. The built-in protection circuit of the battery pack should serve a safe purpose when the battery is in an abnormal working state, thereby reducing the degradation of the lithium-ion battery.
3. Short circuit battery
The suspected reason is that the battery may have been mixed with foreign particulate impurities during the manufacturing process. Even the top 10 lithium battery companies in the world often fail to explain why some batteries exhibit high leakage rates or electrical short circuits when they are still in newer condition.
The other is that rough spots on the electrode cause damage to the diaphragm. Therefore, the battery manufacturing process should be improved, which can greatly reduce the lithium ion battery degradation.
- A polarity reversal of the battery caused by deep discharge can also cause a short circuit in the battery.
- High reverse currents can cause permanent electrical short circuits.
- Another cause is diaphragm damage caused by the formation of uncontrolled lenses, which is known as the memory effect.
The use of instantaneous high current pulses to attempt to repair short-circuited batteries has an extremely limited success rate. This short circuit may temporarily evaporate, but the damage to the diaphragm material remains.
This repaired battery often exhibits a high discharge rate and the short circuit will reappear. It is not advisable to replace a short-circuited battery in a degraded lithium ion battery pack. Unless the new battery matches the performance of the other cells in the battery pack in terms of battery voltage and capacity.
4. Loss of electrolyte
Although the batteries are all sealed, they lose some electrolyte during the lithium ion battery degradation. Once gas emissions occur, white powder accumulates around the gasket, and the loss of electrolyte will eventually reduce battery capacity.
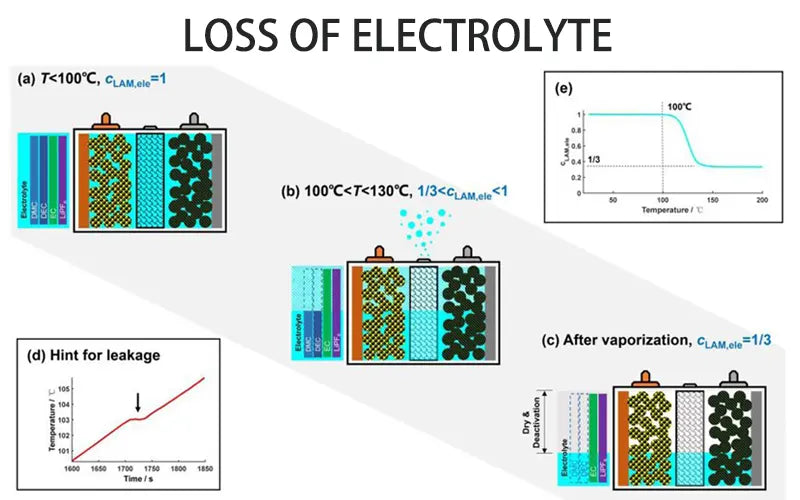
Loss of electrolyte in lead-acid batteries is a long-standing problem. The reason for this is overcharging and working at high temperatures. The loss effect of supplementing electrolytes with water is limited. Although battery capacity can be partially restored, battery performance will not be very reliable.
If charged correctly, lithium ion batteries should not have gas problems that cause exhaust problems. The top 5 battery electrolyte companies use the right materials in the production process to avoid these situations. However, lithium ion batteries also experience internal pressure under certain conditions.
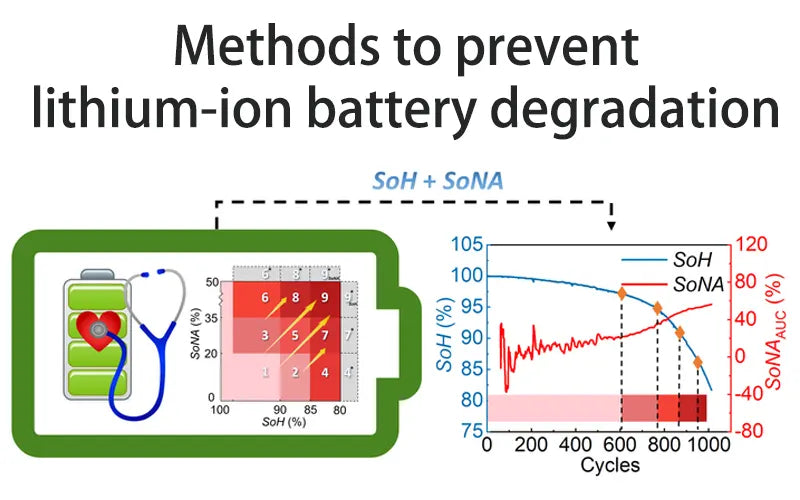
5. Methods to prevent lithium ion battery degradation
The graphite anode currently used in commercial lithium ion batteries has a low actual capacity at low temperatures. Hard carbon has good low-temperature lithium storage capacity, which can effectively improve the lithium ion battery degradation. However, in the process of battery formation, hard carbon anodes will irreversibly consume Li+, thereby reducing the actual capacity and specific energy of the battery.
Graphite has innovatively developed composite electrodes to create a lithium ion aqueous full battery with an energy density of 460Wh/kg and a coulomb efficiency of about 100%. Based on the conversion reaction combined with high energy density, the battery has excellent intercalation reversibility, which improves the safety of aqueous batteries.
A solvent mixture in the electrolyte of a lithium ion battery, including dissolved lithium salt, and an organic additive. It can increase the surface and overall stability of the silicon anode, improve the long-term cycle and service life and lithium ion battery degradation.
Cyclohexanohexanone, an organic cathode material for ultra-high capacity lithium ion batteries, has a specific discharge capacity of up to 902mAhg-1. In addition, due to the low solubility of cyclohexanone in highly polar ionic liquids, it has better cycling performance in ionic liquid-based electrolytes, and the lithium ion battery degradation characteristics are few.
There is a risk of short circuits and fire inside the lithium ion battery. However, boron nitride nanofilms of 5 to 10 nanometers can be used as a protective layer to isolate the electrical contact between lithium metal and electrolyte.
Boron nitride nanofilm is chemically and mechanically stable to lithium, and has a high level of electronic insulation, so it can greatly improve the safety of lithium ion batteries.
6. Conclusion
With the development of science and technology and the maturity of technology, lithium ion batteries are more and more widely used. Lithium ion batteries have the advantages of high monomer voltage, relatively light weight, and environmental friendliness。
But after multiple charge-discharge cycles, it will cayse lithium ion battery degradation. The faster the battery capacity decays under the same conditions, the lower the battery quality. Improving the performance of lithium ion batteries is an important indicator to measure their quality.
Related articles: battery self discharge, lithium ion battery life, Lithium ion battery overcharge
















