Main content:
In battery modules consisting of pouched lithium-ion batteries, battery stacking is a common structural form. Stacked pouch cells need to withstand a certain amount of pressure to place the necessary constraints on the batteries body.
During the research process, it was found that under pressure, the wound pouch lithium-ion battery will cause battery electrode crack after multiple charges and discharges. In this paper, the mechanism and protection method of the fracture phenomenon of the pole will be studied.
1. Reasons for the charge and discharge process
The battery electrode crack is related to the expansion of the battery anode material and external pressure. Stress is generated in the process of charging and discharging.
When lithium ions are embedded in the cathode or anode, uneven lithium ion concentration may lead to stress generation. Therefore, tensile stress on the electrode surface leads to the propagation of cracks, which leads to the degradation of the mechanical electrode material.
● Expansion of the anode material
Since lithium is embedded or detached from the active material in the electrochemical cycle, the stress caused by diffusion is the main cause of battery electrode crack and fatigue failure, so changes in the internal stress of the batteries may cause swelling.
In general, during lithium intercalation and deintercalation, mechanical stress due to volume expansion may occur in the electrode. In addition, repeated volume expansion leads to periodic stress of the electrode material during cycling.

Cyclic charging and discharging of graphite composite electrodes in lithium-ion batteries leads to periodic volume changes in the electrode, resulting in electrode degradation and capacity decay. During charging and discharging of lithium-ion batteries, the anode and cathode materials expand and contract as they are embedded or deintercalated.
● External pressure
The pressure of the lifepo4 batteries and the pressure generated by the expansion during the charging and discharging process have a great influence on its performance.
Different pressures are applied to the pouch lithium-ion batteries to charge and discharge, and the batteries segment cracks after 2000 cycles of the stressed battery. The unpressurized battery anode sheet produces a large amount of lithium precipitation.
The battery under pressure can not only avoid the battery electrode crack, but also inhibit the phenomenon of lithium evolution in the anode to a certain extent. Next, the mechanism of the battery electrode crack of the lithium ion pouch under pressure is analyzed. And the protection method of this is obtained, which provides a basis for the design of the whole module.
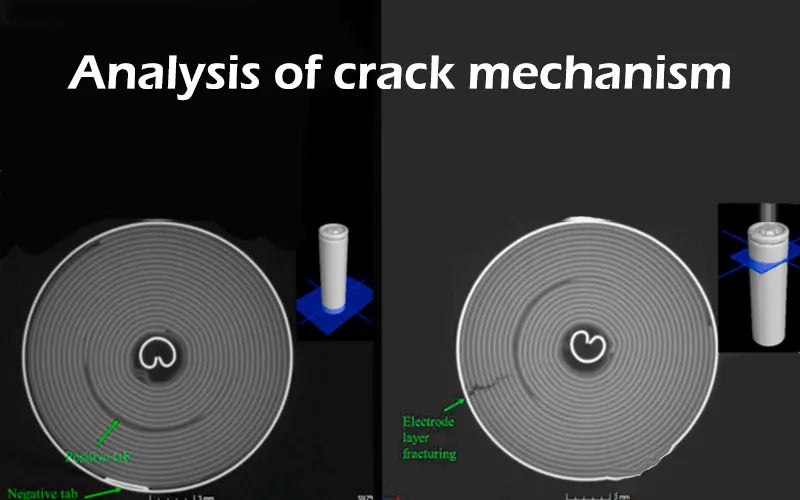
2. Battery electrode crack phenomenon
Under the winding structure of the lithium-ion battery voltage, and after 2000 charge and discharge cycles, the battery electrode crack. Summarizing the characteristics of the battery electrode crack phenomenon, it is found that most of the battery electrode crack are at the first bend of the anode electrode.
The substrate material of the cathode sheet is aluminum foil, and the substrate material of the anode sheet is copper foil, which is used for conduction inside the lithium batteries. The cathode and anode materials are coated on the base material respectively, and the cathode and anode tabs are welded to the innermost layer of aluminum and copper foil.

When the copper foil coated with the anode material cracks, the internal structure of the battery changes, which will affect the performance of the batteries. Therefore, the battery electrode crack is a problem that cannot be ignored.
3. Analysis of crack mechanism
Lithium ion batteries are mainly composed of a cathode, a anode, an electrolyte and a separator. The cathode material of ternary lithium-ion batteries is nickel-cobalt-manganate, lithium Li (NiCoMn)O2.
The crystal structure is stable, and lithium ion intercalation has little effect on lattice volume during charging and discharging processes. The electrolyte and separator make up a small proportion of the batteries and are inherently compressible. Therefore, the influence of lithium ion deintercalation on anode graphite is mainly considered.
● Ideally
When charging, lithium ions come out of the cathode and are uniformly embedded in the anode graphite, and when discharging, lithium ions come out of the anode graphite and are embedded in the cathode through the diaphragm.
In the extraction process, the internal structure of the original layered graphite is not affected, and the gap inside the graphite is completely elastic, so under ideal conditions, the lithium-ion batteries expand and contract every cycle, and the volume remains unchanged.
● In practice
When the battery is charged, the transport channel changes all the time. When discharging, the position of Li+ coming out of the graphite is different from the position embedded in the charging time, the amount of Li+ embedded and detached is not equal, and the internal gap of the layered graphite is inelastic.
When Li+ is embedded in anode graphite, it will destroy the original balance of force between graphite layers, and increase the repulsion between atoms, and graphite will expand.
When Li+ comes out of the anode graphite, the graphite will shrink again, but the overall amount of expansion is more than the contraction, which ultimately leads to an increase in the irreversible expansion of the anode of each charge and discharge ring.
During the charging process of lithium-ion batteries, the volume of the batteries will expand to a certain extent due to the expansion of graphite, but the expansion at this time is uniform, so there will be no breakage of the batteries. If the internal stress of the battery increases, the material on the anode sheet will expand to the side, and the copper foil will expand to the outside.
The greater the pressure applied to it, the more the copper foil is stretched outward, and after two or three thousand charge and discharge cycles, the copper foil is stretched beyond the tensile fatigue strength, resulting in the fracture of the batteries. It is deduced that the battery electrode crack is the result of the combined effect of graphite expansion phenomenon and external pressure.
4. Protection method under pressure state
● Reduce pressure
According to the analysis results of the fracture mechanism of the battery electrode crack, The top 10 lithium ion battery anode material companies have worked out the simplest macroscopic protection scheme: to reduce the pressure on itself.

However, lithium-ion batteries must withstand certain pressures in the application of group structures. In addition, proper pressure is good for battery life.
● Adjust the pressure distribution
The basic purpose of reducing pressure is to reduce the expansion of the batteries to the side. That is, the pressure on both sides of the battery needs to be reduced.
At the same time, most of the middle area of they needs a certain pressure to ensure battery life, and one solution is to design a pressure adjustment device. It is added between the batteries so that the pressure in the middle part of the batteries is large, and the pressure on both sides is small.
The elastic silicone pad is made of silicone material, which has good elasticity and recovery, suitable softness during pressure changes and temperature fluctuations, and can fit well with the contact surface.
5. Conclusion
Aiming at the problem of fracture of fragments after multiple charges and discharges of pouch lithium-ion batteries under pressure, two solutions are proposed to reduce the pressure and adjust the pressure distribution.
Reducing the pressure on the batteries can effectively prevent the battery electrode crack. Through this article, the cause is found and an effective method to prevent the anode from cracking is verified.
Related articles: car battery voltage, Top 10 lithium battery electrolyte companies, lithium ion battery electrolyte