Main content:
1.Introduction to hydrogen storage

Hydrogen is the most abundant element on Earth, and along with carbon and oxygen, it builds stable basic chemical reactions to provide energy. As natural energy sources are nearly depleted or difficult to extract, hydrogen is considered to be an alternative to carbon and an efficient energy source for future vehicles.
Moreover, hydrogen-fueled clean energy vehicles are considered plausible and can address the impact of greenhouse gases. This is because the only product of hydrogen combustion is water, which helps to prevent the greenhouse gas effect caused by carbon dioxide emissions, which are increasing due to increased human activities. As a result, hydrogen is widely believed to be an important player in the energy sector in the coming centuries - replacing fossil fuels, although the latter currently dominate due to advantages such as abundance, ease of storage and use, and initial cost. The advantages of fossil energy are also the competitive standards that hydrogen will need during this period of transition, research and optimization. In any case, the development process of hydrogen will parallel its competitors, after all, energy needs and application forms are diverse.
Before the era of industrial development and economical application of hydrogen, some key technical issues still need to be solved, which run through the four links of the energy life cycle: production, storage, distribution and use (PSDU). There are obvious dependencies between each link, and the final solution can only be determined from the overall interests of the PSDU system. For example, hydrogen produced in different ways will not be stored in the same way, and various chemical or physical storage methods also determine different redistribution methods. Likewise, how fast or slow hydrogen burns requires different techniques to handle.
Research on hydrogen storage has made important progress in the past few years, depending on the physical or chemical state, storage in gaseous, liquid or solid form (depending on whether it is atomically or molecularly bonded). There are many parameters that play a role in it. Here are a few important indicators, including density, specific density, application scale, reaction speed, reversibility, safety and system economy. There is no uniform standard. The possibility is related to System requirements vary widely.
Before giving a general introduction to the current state of hydrogen storage technology, we first review the basic physical, thermodynamic, and chemical properties of hydrogen in different forms, which will affect all aspects of hydrogen. In the following three subsections, we will introduce the latest research progress on hydrogen storage, including gaseous form, liquid form, solid form (bonding).
We will discuss the advantages and disadvantages of each of these hydrogen storage methods in accordance with the PSDU section of hydrogen.
2.Overview of Hydrogen Storage
2.1Relevant energy parameters for hydrogen storage for hydrogen storage
The energy performance of hydrogen and conventional fuels was compared. The obvious advantage of the complex is that the oxidation process (combustion) produces four times the energy of the carbon oxidation process and, unlike fossil fuels, the combustion process does not produce carbon dioxide. It is also important to consider the form of application, such as whether the combustion product of hydrogen, water, is in steam or liquid form.
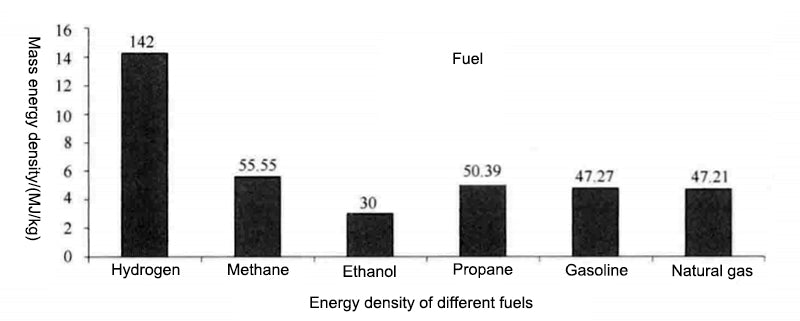
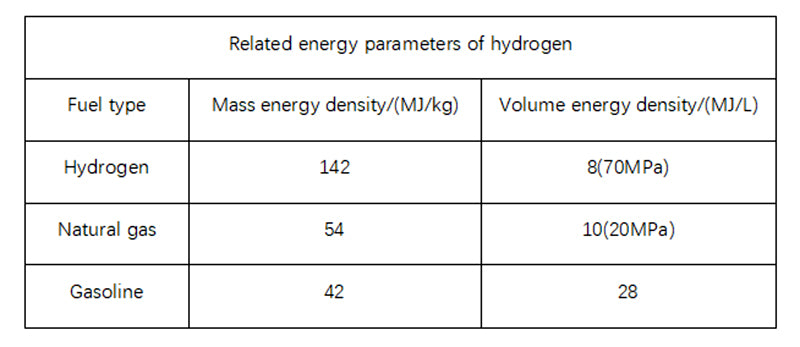
The data above were obtained under the most favorable conditions (liquid water), if under other conditions, the energy density of hydrogen would be reduced to below 125 MJ/kg (1kg H2 = 33.33 kW h).
However, the data presented in the table does not reveal the major drawback of its particularly low volumetric energy density compared to conventional fuels. Under normal conditions, 1 kg of hydrogen occupies more than 11m3 of space. As a result, in the hydrogen storage process, the compression of hydrogen becomes an important link.
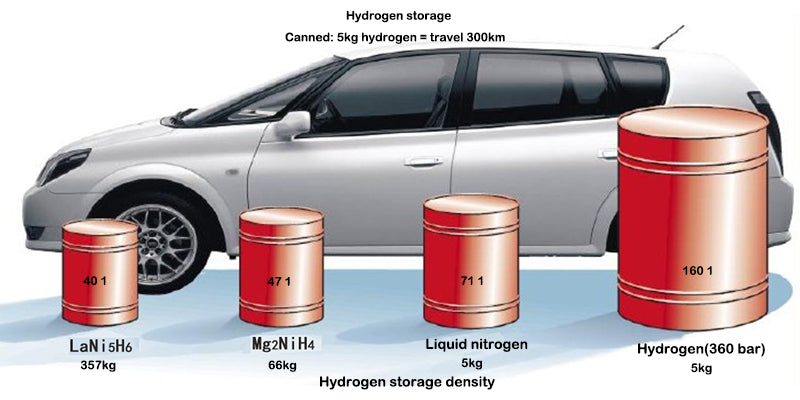
2.2Density and specific density for hydrogen storage
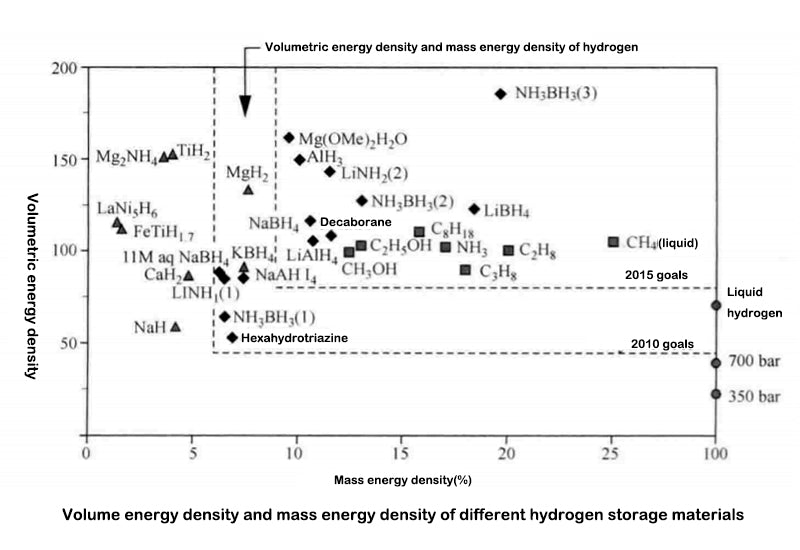
The diagram below gives the volume and mass of the three main forms of compressed hydrogen storage: the physical and molecular states (i.e. high pressure, gaseous hydrogen or cryogenic liquid hydrogen), the solid or "atomic state" (chemical state) stored as metal hydrides , or adsorbed to other materials (molecular state).
The physical form of hydrogen storage (under pressure or low temperature) in the diagram below does not take into account the mass of the hydrogen storage vessel. But in practice, these parameters are very important and will be discussed in the corresponding section of each storage mode.
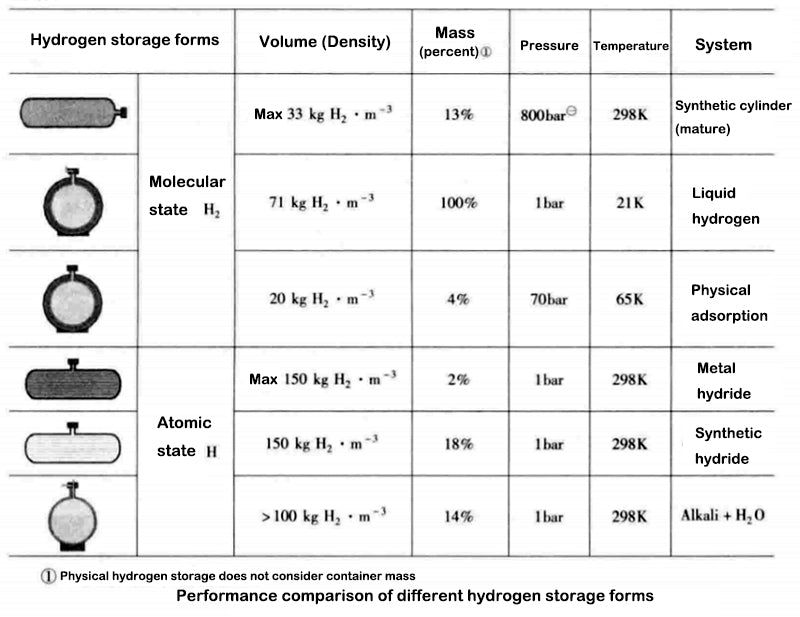
The above figure shows the advantages and disadvantages of different hydrogen storage forms. For most metal hydrides, the hydrogen absorption and release processes are more efficient at low pressures. The chemical reaction of the discharge is an endothermic process, but this is considered a safe feature (such as in the event of a hydrogen leak).
The data in the figure below comes from the U.S. Department of Energy, but only gives the hydrogen storage capacity of the material itself, without considering the quality of the container, nor the quality of water necessary for the recovery of compounds such as borides. Note that the mid-2010-2015 target phase in the graph includes only one "traditional" metal hydride; after 2015, only borides, aluminum oxides, and other complex hydrides will be recognized by DOE experts . Chemical or physical risks are not included, no reference is given to (extraction and production) costs, nor is there a description of the natural resource holdings of the relevant elements. Therefore, it is difficult to directly use the data in the graph as a basis for practical application and setting near-term goals. Therefore, the role of this graph is limited to comparing the hydrogen storage capacity of different materials.