
main content:
The electrolyte lithium salt is not only the provider of lithium ions in the electrolyte, but its anion is also the main factor that determines the physical and chemical properties of the electrolyte. Studies have shown that solution impedance, surface impedance and charge transfer impedance all depend on the composition of the electrolyte. Lithium salts mainly used in lithium-ion batteries, such as lithium perchlorate (LiClO4), lithium hexafluoroarsenate (LiAsF6), lithium tetrafluoroborate (LiBF4), lithium trifluoromethanesulfonate (LiCF3SO3), lithium hexafluorophosphate (LiPF6) They all have larger anions and low lattice energy. LiClO4 has been widely used in experimental batteries. Because LiClO4 is a strong oxidant, it may cause safety problems under certain uncertain conditions, thus affecting its practical application; As As is toxic and LiAsF6 is expensive, the application of LiAsF6 is also restricted; LiBF4 The conductivity of LiCF3SO3 in organic solvents is low, and LiCF3SO3 corrodes the aluminum electrode of the positive current collector. LiPF6 is a conductive lithium salt that is widely used in lithium-ion batteries. The electrolyte containing LiPF6 can basically meet the requirements of lithium-ion batteries for the conductivity and electrochemical stability of the electrolyte. However, LiPF6 is complex to prepare, has poor thermal stability, and is easily exposed to water. Decomposition, expensive, etc. Comparing the electrochemical stability of the EC/PC solutions of LiClO4, LiBF4 and LiPF6, as the electrolyte of LiBF4 has the lowest charge transfer resistance and surface film resistance, the solution containing LiBF4 is the most stable of the three solutions, so the electrode of LiBF4 The impedance is the lowest. HF plays an important role in LiPF6 solution, and the deposition electrode impedance of LiF is relatively high.
One aspect of the research on lithium salt is to modify LiPF6, such as substituting all six F atoms with catechol groups to obtain lithium tris(catechol)phosphate which is not easy to be hydrolyzed and has good thermal stability. However, the anion of the salt is relatively large, so the electrolyte containing the salt has a high viscosity, low conductivity, and an oxidation potential of only 3.7V. Another type of lithium salt is to partially replace the F atom with a CnF2n+1 group with strong electron withdrawing ability to obtain a series of LiPF6-m(CnF2n+1)m. The biggest advantage of this salt is that it does not hydrolyze, but its preparation process is extremely complicated, and its conductivity is low compared with LiPF6.
On the other hand, it is looking for a new type of organic electrolyte lithium salt that can replace LiPF6 with better performance. The basic idea is that the organic anion of the lithium salt is composed of two parts A and B. The A part takes the atoms of boron, carbon, nitrogen, aluminum and other elements as the central atom, and the B part can disperse the charge and stabilize the electrochemical performance of the lithium salt. Strong electron withdrawing groups, such as Rf, RfO, RISO3, RfSO2, RfCO2 or bidentate ligands like oxalic acid. According to the molecular structure, it can be divided into the following categories.
1. Lithium salt of C central atom
For example, LiC(CF3SO2)3 and LiCH(CF3SO2)2, etc., LiC(CF3SO2)3 has relatively high thermal stability, and LiCH(CF3SO2)2 has relatively stable electrochemical performance.
2. Lithium salt of N central atom
Such as two (trifluoromethylsulfonyl) imide lithium LiN (CF3SO2)2 (abbreviated as LiTFSI). Due to the highly delocalized dispersion of the anionic charge, the salt is easily dissociated in the organic electrolyte, and its conductivity is equivalent to that of LiPF6, and it can also form a uniform passivation film on the surface of the negative electrode, but this salt starts at around 3.6V It has a strong corrosive effect on the positive electrode current collector aluminum foil, so it is not suitable for use in lithium ion batteries using aluminum as the current collector. LiN(C2F5SO2)2, an imine lithium salt with long fluoroalkyl groups, will not corrode the aluminum electrode at 4.5V and LiN(CF3SO2)(C4F9SO2) at 4.8V. They can form a good passivation film on the surface of the aluminum electrode. The structure of lithium bis(polyfluoroalkoxysulfonyl)imide [LiN(ROSO2)2] is similar to that of lithium bis(polyfluoroalkylsulfonyl)imide, and its substituent is not polyfluoroalkyl Rf, but Polyfluoroalkoxy RfO. Its chemical stability and thermal stability are higher, and its electrochemical window is wider than LiTFSI. For example, the oxidation potential of LiN[SO2OCH(CF3)2]2 is as high as 5.8V, but its conductivity is lower than LiTFSI.
3. Lithium salt of B central atom

Mainly lithium borate complexes, such as chelated lithium borate salts. Generally adopted formula:
LiOH+B(OH)3+2R(OH)2→Li[B(RO2)2]+4H2O
Their structure is shown in Figure 4.
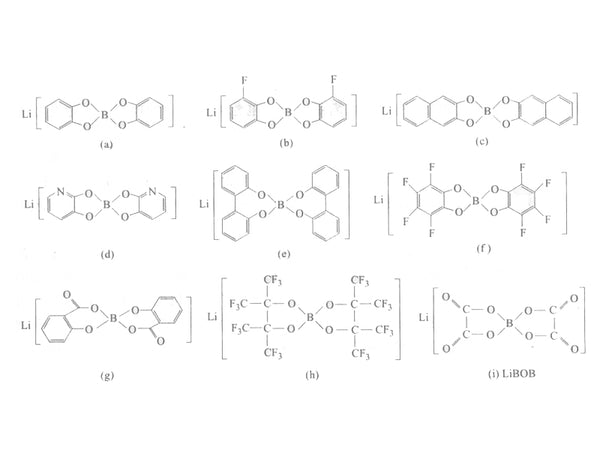
Figure 4 Schematic diagram of the structure of lithium borate
Such salts generally have larger anions, their solubility is higher, and their electrochemical stability is higher. In Figure 4 (a) to (i), the electrochemical windows of each salt relative to metallic lithium are 3.6V, 3.75V, 3.8V, 3.95V, 4.1V, 4.1V, 4.5V, >4.5V and >4.5V.
Lithium bis(perfluoropinnacle) borate [ie (h) in Figure 4] not only has high thermal stability, but also high electrochemical oxidation stability, with an electrochemical window of 5V. From the structural point of view, it contains 8 strong electron withdrawing groups CF3, which can make the charge on B highly dispersed, so it has good conductivity. The room temperature conductivity of lithium bis(perfluoropinnacle) borate in DME can reach 11.1×10-3S/cm. In addition, lithium bis (oxalato) borate [lithium bis (oxalato) borate, LiBOB] has a decomposition temperature of 320°C, high electrochemical stability, and a decomposition voltage >4.5V. Because the negative charge on B is affected by the surrounding eight Oxygen atoms are highly dispersed, and this salt has greater solubility in most common organic solvents. In addition, compared with the traditional lithium salt, it has two significant advantages: ①The lithium ion battery using LiBOB electrolyte can work at high temperature without capacity degradation; ②In the pure solvent propylene carbonate (PC), use LiBOB electrolyte batteries can still be charged and discharged normally and have good cycle performance. The BOB- anion can participate in the formation of the SEI film on the surface of the graphite anode material, forming an effective SEI film, preventing the solvent and solvated lithium ions from intercalating between the graphite layers, so that it can be effective regardless of the high temperature or the presence of PC Stable graphite anode.
There are two kinds of lithium borates with two oligoether chains and electron withdrawing groups directly bonded to the complex center of the ester, which are CF3COO- or C6F5O-. Salt A with a length of 7.2 EO repeating units at 30°C, as shown in Figure 5, has a maximum ionic conductivity of 4.5×10-5S/cm. Due to the weaker combination of lithium ions and oxygen atoms on the borate, the lithium borate has a higher conductivity than the corresponding aluminate. The mobility of ions in the lithium salt is related to the motion of the EO chain segment, and the motion conditions of the ions can be described by the parameters σ0 and B value of the VTF equation. It can be seen from the comparison of the σ0 value that lithium borate has a larger number of carriers than lithium aluminate. Both Salt A and Salt B have a higher lithium ion migration number than the PEO-LiX system, as shown in Table 1.
| Lithium borate | Lithium ion migration number |
| Salt A (n=3) | 0.68 |
| Salt A (n=7.2) | 0.76 |
| Salt A (n=11.8) | 0.82 |
| Salt B (n=3) | 0.62 |
| Salt B (n=7.2) | 0.70 |
| Salt B (n=11.8) | 0.75 |
Table 1 Lithium ion migration data of lithium borate at 70℃
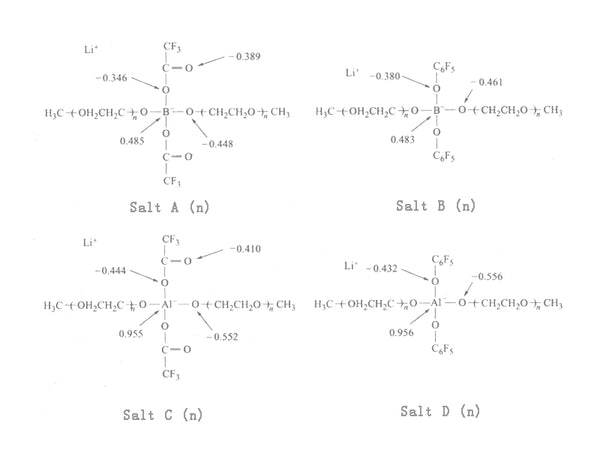
Figure 5 Schematic diagram of part of the charge structure in lithium borate and lithium aluminate
4. Lithium salt of Al central atom

Aluminum and boron are elements of the same family, and there are many similarities in chemical properties. Using aluminum atoms to replace boron atoms in lithium borate compounds, you can get lithium aluminate, such as lithium aluminate LiAl[OCH(CF3 )2]4, not only shows high thermal stability and electrochemical stability, but also has high solid state conductivity and low melting (mp=120℃). In the molten state, the self-diffusion coefficient of lithium ions is much higher than the self-diffusion coefficient of anions. LiAlCl4·xSO2 is used as the electrolyte of Li/C and Li/CuCl2 in secondary batteries, and dry SO2 gas can be passed into LiAlCl4 to obtain dark brown LiAlCl4·3SO2 liquid, where x can be quantitatively controlled from 3 to 12. In the range of -10~+50℃, the conductivity of the electrolyte LiAlCl4·3SO2 is (70~130)×10-3S/cm. Taking into account the oxidation and decomposition of SO2, the open circuit voltage of the rechargeable battery with LiAlCl4·xSO2 as the electrolyte is set between 3.3V and 3.5V, and the discharge platform of the battery is around 3.2V. Electrolyte overcharge protection mechanism: In the overcharged state, LiAlCl4 is oxidized to produce Cl2, and then Cl2 reacts with Li to form LiCl, and LiCl recombines with AlCl3 in the solution to form LiAlCl4. Since the Cl2 formed in the reaction has a corrosive effect on the polypropylene diaphragm, glass fiber is used as the diaphragm.
5. Ionic liquid/room temperature molten salt electrolyte

Ionic compounds are generally solid at room temperature, and strong ionic bonds make the anions and cations bound to the crystal lattice only for vibration, but not for rotation or translation. Due to the strong Coulomb effect between anion and cation, ionic crystals generally have higher melting points, boiling points and hardness. If the anions and cations are made large and the structure is asymmetric, then due to the influence of steric hindrance, the strong electrostatic force cannot make the anions and cations densely packed on the microscopic level, the interaction between the ions is reduced, and the lattice energy is reduced. In this way, the anions and cations can not only vibrate at room temperature, but can even rotate and translate, destroying the order of the crystal structure and lowering the melting point of the ionic compound. The ionic compound may become liquid at room temperature. It is usually called room temperature molten salt. Because this liquid is completely composed of anion and cation ions, some people call it an ionic liquid. Room temperature molten salt has the following advantages:
①It is liquid in a wide temperature range. Most molten salt can maintain liquid state at -96~200℃, such as water at 0~100℃ and ammonia at -77~-33℃ as liquid;
②High thermal stability, can reach 200℃ without decomposition;
③The vapor pressure is very low, almost 0;
④Exhibit Bronsted, Lewis and Franklin acidic and super acidic properties;
⑤It can dissolve a wide range of organic, inorganic, polymer substances, and even rocks (but not polyene, PTFE or glass), and it is an excellent solvent;
⑥ Non-flammable and non-corrosive.
Due to the above advantages of room temperature molten salt, it is known as the "green solvent". Room temperature molten salt is used in electrochemistry, photochemistry, organic reaction media, catalysis, separation and purification, biochemistry and liquid crystal, etc. In addition, due to the high conductivity of some room temperature molten salts, wide electrochemical window, and their non-flammable and non-volatile characteristics, they have become promising safe electrolytes for applications in high energy density batteries and photoelectrochemical solar cells. , Electroplating and super capacitors, etc.
The literature records of room temperature molten salt can be traced back to 1914. Sudgen et al. reported that the salt was liquid at room temperature, ethylamine nitrate, with a melting point of 12°C, but it did not attract people's attention at that time. In 1951, Hurley et al. reported a room temperature molten salt of chloroaluminate, AlCl3-N-ethylpyridine bromide, and used the molten salt for metal electrodeposition. In 1979, Osteryoung et al. reported a room temperature molten salt system formed by AlCl3 and N-butylpyridine chloride, and found that the melting point of the system was lower than room temperature in the range of molar ratio of 0.75 to 2.0. In the same year, Hussey et al. systematically studied the various physical and chemical properties of the molten salt formed by alkylpyridine chloride and AlCl3. In 1982, they reported a new room temperature molten salt based on AlCl3 and 1-methyl-3-ethylimidazole chloride, which has similar properties to the alkylpyridine molten salt system, but has a higher conductivity 2 ~3 times, the viscosity is about half lower, and the electrochemical window is obviously better than that of alkylpyridines. When the molar ratio of AlCl3 to EMICI is 2:1, the system exhibits the lowest melting point -75°C.
The disadvantages of room temperature molten salt are: it is sensitive to air and water, easy to absorb moisture in the air, and is not conducive to operation. Therefore, many new molten salt systems that are not sensitive to air and water have been developed later. In 1997, the molten salt tetrafluoroborate 1-methyl-3-ethylimidazole (EMIBF4), which is insensitive to water, appeared, and then the hydrophobic hexafluorophosphate 1-methyl-3-ethylimidazole (EMIPF6) was synthesized. come out. In the following years, various quaternary ammonium salts, quaternary zinc salts, alkylpyridines and alkylimidazoles organic cations (Figure 7) and NO3-, ClO4-, BF4-, PF6-, CH3COO-, CF3COO-, CF3SO3-, N(CF3SO2)2-, N(C2F5SO2)2-, C(CF3SO2)3- and other anions composed of room temperature molten salts have been synthesized one after another, but the structure of these room temperature molten salts organic cations is more complex, synthetic preparation It is relatively difficult to purify, so the researchers turned their attention to a system with a relatively simple structure. In 1999, Hirao et al. directly neutralized various tertiary amines with tetrafluoroborate to prepare a series of proton conductor room temperature molten salts. Among them, tetrafluoroborate 1-methylpyrazole has the lowest melting point (-109.3°C) and conductivity The highest (1.9×10-2S/cm). In 2002, Hagiwara et al. reacted 1-methyl-3-ethylimidazole chloride with anhydrous hydrofluoric acid to prepare a new room temperature molten salt EMIF·2.3HF, which It has a very high room temperature conductivity (0.1S/em). In 2003, someone prepared a room temperature molten salt of a proton conductor based on organic amines and inorganic or organic acids, and studied various physical and chemical properties of the system, including electrical conductivity , Viscosity, vapor pressure, etc., to obtain a system whose conductivity is comparable to that of an aqueous solution.
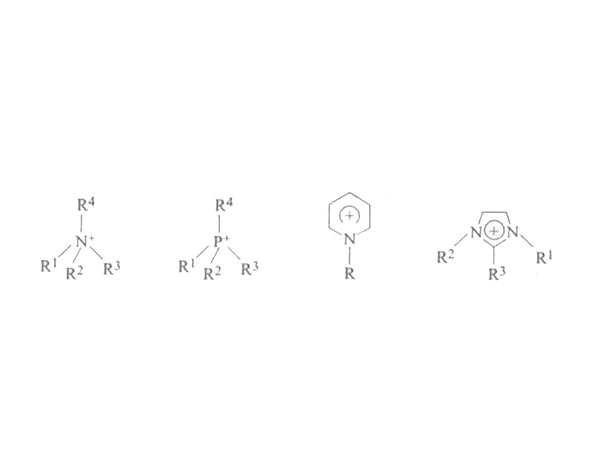
Figure 7 Schematic diagram of the structure of organic cations in room temperature molten salt
Another type of room temperature molten salt composed of amide and alkali metal nitrate or ammonium nitrate, such as urea (59.1%, mole fraction)-ammonium nitrate (40.9%, mole fraction) (mp=63.5°C), urea-acetamide- Ammonium nitrate (mp=7℃), a room temperature eutectic salt formed by urea-acetamide-alkali metal nitrate appeared in 1993. It has good conductivity and an electrochemical window of about 2V, but the system is unstable and easy Crystals precipitated. Urea can form low-temperature molten salts with lithium perchlorate, lithium thiocyanate, sodium thiocyanate, lithium chloride, etc., and some systems have higher room temperature conductivity. The author’s laboratory has also prepared a binary room temperature molten salt based on lithium tris(trifluoromethanesulfonyl) ammonium (LiTFSI) and urea. The melting point of LiTFSI is 234°C, and the melting point of urea is 132.7°C, but mixing the two in a certain ratio can slowly spontaneously form a liquid at room temperature. But its room temperature conductivity is low (1.74×10-4S/cm), and its viscosity is too large (1780mPa·s)
The room temperature molten salt of organic aluminum trichloride can be used as the electrolyte of the battery, and the dual-cell molten salt battery (DIME) is a typical example of using molten salt as the electrolyte. The positive and negative electrodes of the battery are both cheap and easily available graphite intercalation compounds, and the molten salt provides both anions and cations, and is inserted into the graphite positive and negative electrodes respectively. The battery uses 1,2-dimethyl-3propylimidazole-aluminum tetrachloride (DMPI-AlCl4-) as the electrolyte, the open circuit voltage is 3.5V, the cycle efficiency is 85%, and the advantage of the battery is to avoid the use of any organic solvents And volatile substances, and the battery can be assembled in a discharged state. The tetrafluoroborate 1,2 dimethyl-4-fluoroimidazole (DMFPBF4) molten salt based on imidazole cations has a thermal stability of up to 300°C, and can coexist stably with lithium in a wide temperature range. The electrochemical window is about It is 4.1V and the oxidation potential is greater than 5V (relative to Li/Li+). Some people use this molten salt as an electrolyte to assemble a LiMn2O4/Li lithium-ion battery, which has a high degree of reversibility (coulomb efficiency greater than 96%). For the N,N-dialkylpyrrolidine molten salt series of the cationic system, when the substituents on the pyrrolidine N are asymmetric, its melting point decreases, and the N,N-dimethyl-n-butylpyrrolidine bis(trifluoro The glass transition temperature of the methylsulfonyl) imide salt is -87℃, the melting point is -18℃, the electrochemical stability window exceeds 5.5V, and the room temperature conductivity is 2.2×10-3S/cm. Lithium ions are doped into such organic In the molten salt system, a type of lithium ion fast ion conductor plastic crystal electrolyte can be obtained. The lithium salt contains the same anion as the substrate, and the lithium salt doping can be considered as a cation substitution. Due to the rotation disorder and the existence of lattice vacancies, the rapid migration of lithium ions is caused. When the substrate and doping ratio are appropriate, the conductivity at 60°C can reach 2×10-4S/cm. LiTFSI is dissolved in N,N-dimethylpropylpyrrolidine bis(trifluoromethylsulfonyl)imide salt As the electrolyte, LiCoO2/Li battery is assembled. The discharge capacity of the battery in the first week is as high as 120mA·h/g, and the charge-discharge efficiency is 97% after the second week. LiCl was dissolved in the room temperature molten salt of AlCl3-chloro-1-methyl-3-ethylimidazole as an electrolyte, and LiCoO2/LiAl battery was assembled. The discharge capacity in the first week was 112mA·h/g, and the charge and discharge efficiency in the first week It was found that adding C6H5SO2Cl to the molten salt at room temperature improved the electrochemical stability of the molten salt and the reversibility of the electrode.
Researchers from the Institute of Physics of the Chinese Academy of Sciences used acetamide, which has a weaker hydrogen bond and a higher dielectric constant and dissociation constant, as the lithium ion ligand. They used bis(trifluoromethylsulfonyl) with similar structures but different anion radii. Lithium imide [LiTFSI, LiN(CF3SO2)2], two (perfluoroethyl) sub[LiBETI, LiN(C2F5SO2)2] and lithium trifluoromethanesulfonate (LiCF3SO3) form a room temperature molten salt, and their The physical and chemical properties were compared and analyzed. Among them, the LiTFSI/acetamide molten salt system shows excellent physical and chemical properties.
The anion radii of the lithium salts LiTFSI, LiBETI and LiCF3SO3 are 0.379nm, 0.447nm and 0.264nm, respectively. It is the difference in the size and structure of their anion radii that determines the slight difference in their performance. If the anion radius is too large, although it is easier to dissociate, it also brings great plastic viscosity to the system. The viscosity of the LiBETI/acetamide system is much higher than that of the LiTFSI/acetamide system, and the liquid phase composition range is relatively narrow. If the anion radius is too small, it is not conducive to the dissociation of the lithium salt. Judging from the structure of the new compound formed by LiCF3SO3 and acetamide with a molar ratio of 1:2, the entire crystal structure is entirely composed of ion pairs formed by Li+ and CF3SO3-. In addition, the CF3SO3-anion has a different structure from the TFSI anion. The SO2 group in TFSI- is surrounded by CF3 groups at both ends, while the SO3 group in CF3SO3- is exposed. Therefore, a stronger hydrogen bond occurs between the oxygen atom in the SO3 group and the NH2 group in acetamide. These two reasons cause the plastic viscosity of the system to be larger and the liquid phase composition range is narrow. Lithium salts with higher lattice energy, such as LiSCN, LiClO4, LiNO3, LiPF6 and LiBF4, can not form stable room temperature molten salts in a wide range of ratios after being mixed and stirred with acetamide.
The melting point of LiTFSI is 234°C, and the melting point of acetamide is 81.2°C. But within a certain range of molar ratio [(1:2)~(1:6)], the two can be mixed at room temperature to form a liquid. The DSC test results show that the lowest eutectic point of the molten salt system is -67℃, and the system with a molar ratio of 1:6 has the highest room temperature conductivity of 1.20×10-3S/cm, and the conductivity at 60℃ reaches 5.73×10-3S/cm. The conductivity of the LiTFSI/NH2CONH2 system (1.74×10-4S/cm) is almost an order of magnitude higher. At the same temperature, within the observed temperature and concentration range, as the concentration of LiTFSI in the system increases, the conductivity of the system decreases; and as the temperature decreases, the conductivity of the high salt concentration system decreases faster. Cyclic voltammetry results show that the oxidation peak potential of the molten salt system on the surface of the Al foil is 2.75V (relative to Li/Li+) in the first cycle, 3.8V in the second cycle, and the oxidation peak shifts after four cycles When the temperature reaches 4.5V, the corrosion of the Al foil by LiTFSI at 3.6V is suppressed, indicating that the molten salt electrolyte reacts irreversibly with Al, forming a stable passivation film on the Al surface. The oxidation peak potential on the Ni foil surface is 4.4V in the first cycle. This room temperature molten salt is applied to a simulated battery with MnO2 as the positive electrode and lithium metal as the negative electrode. The discharge capacity of the battery in the first cycle is 243mA· h/g, which is 80% of theoretical capacity. After completing the first discharge, the MnO2 electrode can still be cycled in the molten salt electrolyte between 2.0V and 3.5V, but the cycle performance of the battery deteriorates. The reason may be that the MnO2 electrode undergoes a structural phase change during the cycle or the SEI film formed by the molten salt electrolyte on the surface of the MnO2 electrode is not stable enough, causing the capacity to decline.
Through the comparison and analysis of the physical and chemical properties of the various series of LiX/RCONH2 room temperature molten salt electrolytes, it is concluded that the room temperature molten salt Lix/RCONH2 with excellent physical and chemical properties can be obtained only when the following conditions are met at the same time:
①RCONH2 is the ligand of lithium ion, its interaction with lithium ion cannot be too weak, otherwise it cannot dissociate the lithium salt, and at the same time, its interaction with lithium ion cannot be too strong, so the ligand of lithium ion and lithium ion The role of the ion should be moderate, not only to ensure the dissociation of the lithium salt, but also to facilitate the migration of lithium ions;
②The hydrogen bond in RCONH2 should not be too strong;
③The size of the anion radius should be moderate;
④ The lattice energy of lithium salt is lower.
The Raman and infrared spectra of the LiCF3SO3/acetamide molten salt system show that because acetamide has two polar groups (C=O and NH2), it can interact with Li+ cations and CF3SO3- anions at the same time. The carboxyl oxygen C=O in acetamide has a strong coordination effect with Li+, which makes the lithium salt LiCF3SO3 dissociate in it, and at the same time destroys the hydrogen bond between the acetamide molecules. The SO3 group in CF3SO3-anion and the NH2 group in acetamide interact through hydrogen bonds. The interaction between cationic solvents, anionic solvents, and cation-anions are broad in a wide range of lithium salt concentrations. exist. The interaction between cation and anion increases with the increase of lithium salt concentration and temperature, while the interaction between cation-solvent and anion solvent decreases with the increase of lithium salt concentration and temperature. It is these interactions that lead to the formation of various ionic structures in the molten salt, such as accumulated ions, ion pairs, and "free" ions.
The interaction and various ionic structures in the LiCF3SO3/CH3CONH2 molten salt system are also proved by the single crystal structure of the new compound LiCF3SO3·2CH3CONH2. Structural analysis shows that the compound LiCF3SO3·2CH3CONH2 single crystal presents an obvious "ladder-like" structure. With lithium ion as the central atom, the chain extending infinitely in the a direction constitutes the skeleton of this "ladder". The hydrogen bond formed between the SO3 group in CF3SO3-anion and the NH2 group in acetamide passes through the acetamide intermolecular The hydrogen bond of, connects the "ladder" and "ladder" in the c direction, forming an infinitely extending plane structure in the a and c directions. In addition, the crystal structure also reflects the chemical environment of the nearest neighbor of the lithium ion, that is, the lithium ion is coordinated with the four surrounding oxygen atoms. Two of the oxygen atoms are derived from the Pu oxygen atoms of the two acetamide molecules, and the other The two oxygen atoms come from the oxygen atoms on the SO3 group in the two nearest neighbors of the CF3SO3-anion.
Quantum chemical calculations and Raman spectroscopy studies show that in the high salt concentration area, the ion structure is mainly composed of accumulated ions; in the medium salt concentration area, the ion structure is mainly dominated by ion pairs; in the low salt concentration area, the ion structure is mainly Dominated by "free" ions.
The relationship between the ionic conductivity and temperature of LiCF3SO3/CH3CONH2 molten salt electrolyte can be well in line with the VTF equation, and the ion transport obeys the free volume model. Under the action of an electric field, the thermal motion of solvent molecules continuously creates some new coordination sites. With the help of the movement of solvent molecules, ions migrate from one coordination site to the next, realizing the directional migration of ions. Ionic conductivity depends on the concentration of various ionic structures in the system, especially as the concentration of "free" ions increases, the conductivity increases.
















