
Lithium, the chemical symbol Li, is in the s zone of the periodic table, alkali metal; atomic number 3; relative atomic mass 6.941 (2). Lithium metal is solid at 298K, and its color is silvery white or gray. In the air, lithium quickly loses its luster.
Lithium is an element of the first period, containing one valence electron (1s22s1), and its density in the solid state is about half that of water. The atomic radius (empirical value) of lithium element is 145pm, the atomic radius (calculated value) is 167pm, the covalent radius (empirical value) is 134pm, the van der Waals radius is 182pm, and the ion radius is 68pm. The chemical properties of lithium are shown in Table 1.
| element | Electronic configuration | Metal radius/nm |
Enthalpy of ionization /(kJ/mol) Level 1 |
Level 12 /×10﹣3 |
Melting point /℃ |
Boiling point /℃ |
E⊖① /V |
﹣△H②diss /(kJ/mol) |
| Li | [He]2s | 0.152 | 520.1 | 7.296 | 180.5 | 1356 | ﹣3.02 | 108.0 |
① Reaction formula Li+(aq)+e=Li(s).
②The dissociation energy of diatomic molecule Li2.
Since lithium has only one valence electron, its binding energy is very weak in a tightly packed unit cell. Lithium metal is very soft and has a low melting point, so lithium-sodium alloy can be used as a refrigerant for nuclear reactors.
The melting point and hardness of lithium are higher than other alkali metals, and its conductivity is weaker. The chemical properties of lithium are inconsistent with other alkali metal chemical properties. The standard electrode potential E⊖① (Li+/Li) of lithium is very low among the same group of elements, which is related to the greater heat of hydration of Li+ (g). When lithium is burned in the air, it can directly interact with nitrogen to form nitrides. This is due to its small ion radius, which makes a greater contribution to the lattice energy. The content of lithium in the lithosphere is very low, mainly in some silicate minerals. The density of lithium is only 0.53g/cm3. Among the alkali metals, lithium has the highest melting point and boiling point, as well as the longest range of liquid range, and has exceptionally high specific heat capacity. These characteristics make it an excellent refrigerant in heat exchange. However, lithium is more corrosive than other liquid metals, and it is often used as a degassing agent for reduction, desulfurization, copper and copper alloys.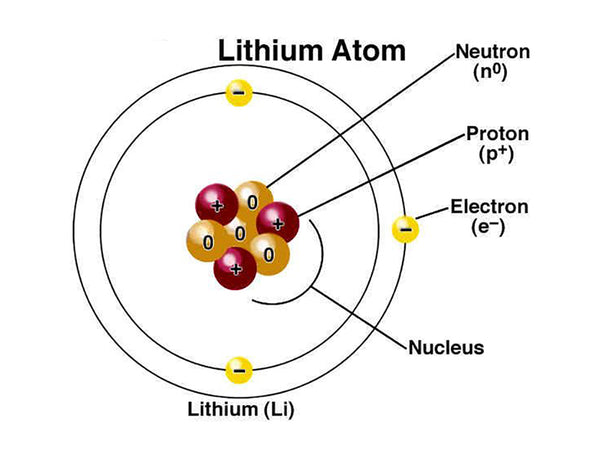
Due to the low ionization enthalpy of the outer layer of lithium electrons, the lithium ion has a spherical shape and low polarity, so the lithium element has a valence of +1. Compared with divalent magnesium ions, monovalent lithium ions have a particularly small ion radius, and therefore have a particularly high charge radius ratio. Compared with other elements of the first main group, the properties of lithium compounds are very abnormal, similar to those of magnesium compounds. These abnormal characteristics are due to the high lattice energy of the lithium salt with low-charge anions, which is particularly stable, while the salt with high-charge and high-valence anions is relatively unstable. For example, the thermal stability of lithium hydride is higher than that of other alkali metals. LiH is stable at 900°C. LiOH is more difficult to dissolve than other hydroxides. Lithium hydroxide decomposes during red heat; Li2CO3 is unstable. Easily decomposed into Li2O and CO2. The solubility of lithium salt is similar to that of magnesium salt. LiF is slightly soluble (0.17g/100g·water at 18°C) and can be precipitated from ammonium fluoride solution; Li3PO4 is hardly soluble in water; LiCl, LiBr, LiI especially LiClO4 is soluble in ethanol, acetone and acetic acid Among ethyl esters, LiCl is soluble in pyrimidine. The high solubility of LiClO4 is due to the strong solubility of lithium ions. High concentration of LiBr can dissolve cellulose. Unlike other alkali metal sulfates, Li2SO4 does not form isomorphous compounds.
The high electrode potential of lithium metal shows its application prospects in batteries. For example, the positive electrode is a lithium sheet, and the negative electrode is a lithium ion secondary battery composed of a composite transition metal oxide material.
Among the elements of the first main group, the activity of reacting with other substances (except nitrogen) increases from lithium to cesium. The activity of lithium is usually the lowest. For example, lithium reacts with water at 25°C, while sodium reacts violently, potassium and water burn, and there is an explosive reaction between rubidium and cesium; with liquid bromine, the reaction between lithium and sodium is moderate. , While other alkali metals react violently. Lithium cannot replace the weakly acidic hydrogen in C6H5C≡CH, while other alkali metals can replace it.
A basic chemical difference between lithium and elements of the same group is the reaction with oxygen. When alkali metals are burned in air or oxygen, lithium generates Li2O, and there is also Li2O2, while other alkali metal oxides (M2O) increase the reaction. Oxide M2O2 and (K, Rb and Cs) superoxide MO2. When lithium is burned in excess oxygen, peroxides are not generated, but normal oxides are formed.
Lithium can directly combine with nitrogen to form nitrides. Lithium and nitrogen react to form ruby-colored crystals Li3N (magnesium and nitrogen form Mg3N2); the reaction is slow at 25°C, and it reacts quickly at 400°C. Using this reaction, both lithium and magnesium can be used to remove nitrogen in the mixed gas. When heated with carbon, lithium and sodium react to form Li2C2 and Na2C2. Heavy alkali metals can also react with carbon, but produce non-quantitative interstitial compounds, which are caused by alkali metal atoms entering the interstitial carbon atoms in the thin-layer graphite.
Both lithium and water react slowly. Lithium hydroxides are all medium-strong bases with little solubility and can be decomposed into lithium oxide when heated. Certain salts of lithium, such as fluoride, carbonate, and phosphate, are difficult to dissolve in water. Their carbonates can be decomposed into corresponding oxides and carbon dioxide under heating. Lithium chlorides are all soluble in organic solvents and exhibit covalent properties.
Lithium forms a series of compounds with amines, ethers, carboxylic acids, alcohols, etc. Among many lithium compounds, the coordination number of lithium is 3-7.
The thermodynamic data of lithium is shown in Table 2.
| state |
△H⊖f
/(kJ/mol) |
△G⊖f /(kJ/mol)
|
△S⊖f /[J/(K·mol)] |
△CP /[J/(K·mol)] |
H⊖298.15﹣H⊖0 /(kJ/mol)
|
| Solid state | 0 | 0 | 29.12±0.20 | 24.8 | 4.632±0.040 |
| Gaseous | 159.3±1.0 | 126.6 | 138.782±0.010 | 20.8 | 6.197±0.001 |
| Gaseous state (Li2) | 215.9 | 174.4 | 197.0 | 36.1 |
Table 2 Some thermodynamic data of metallic lithium
See Table 3 for the relevant data of the crystal structure of lithium.
| Space group | Im-3m (space group number: 229) |
|||||
| structure | bcc (body-centered cubic) | |||||
| Cell parameters |
a/pm 351 |
b/pm 351 |
c/pm 351 |
α/(°) 90.0 |
β/(°) 90.0 |
γ/(°) 90.0 |
The body-centered cubic structure (bcc) of lithium metal is the most stable under the condition of 298K (25℃). Generally, all elements of the first main group (alkali metal) are based on the bcc structure; the shortest distance between Li-Li atoms It is 304pm and the radius of lithium metal is 145pm, indicating that the distance between lithium atoms is smaller than that between potassium atoms. In the bcc unit cell, each lithium atom is surrounded by the eight nearest lithium atoms.
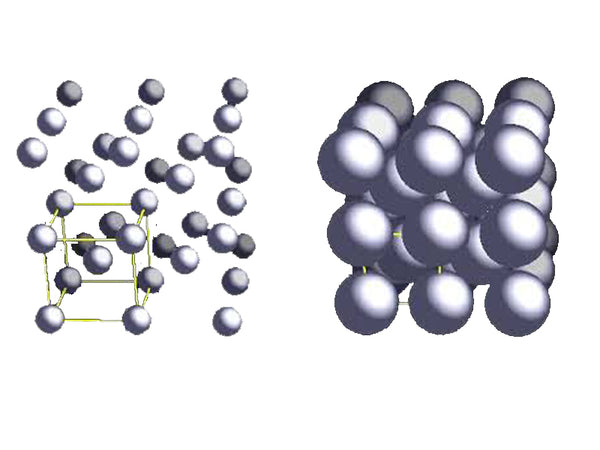
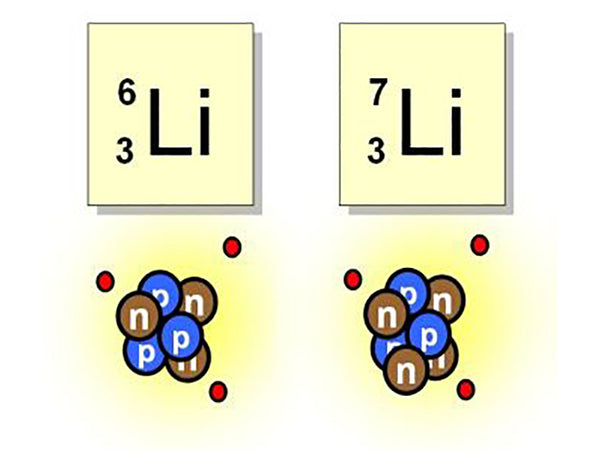
The biggest use of lithium is that it can provide a new type of energy. For example, several isotopes of lithium, 63Li and 73Li, are easily bombarded by neutrons and "fission" to produce another substance, tritium. This type of reaction uses high-speed particles (such as protons, neutrons, etc.) or simple atomic nuclei ( Such as deuteron, helium nucleus) to bombard an atomic nucleus, causing a nuclear reaction, such as:
This reaction means bombarding 63Li with neutrons to generate tritium and helium.
Tritium can release a huge amount of energy in thermonuclear fusion reactions. Lithium is used as core refrigerant in nuclear fusion or nuclear fission reactors. For example, more than 80% of the energy generated in the deuterium-tritium fusion reaction is released in the form of neutron kinetic energy; lithium has a low melting point, a high boiling point, and a large heat capacity and thermal conductivity. Therefore, the liquid lithium is absorbed in the core of the reactor to absorb neutron energy, and then circulated through the heat exchanger to turn the water into steam, which drives the turbine generator to generate electricity. In addition, when the neutrons irradiate the lithium, tritium is generated, and it is continuously multiplying tritium. In the process, lithium is an essential thermonuclear reactor fuel. The so-called hydrogen bomb explosion is this kind of nuclear fusion reaction. According to calculations: 1kg of lithium has the energy equivalent to about 20,000 tons of high-quality coal, which can generate at least 3.4 million kilowatt-hours of electricity, and a 1 million kilowatt power station consumes only 5 tons of lithium a year.
The density of alkaline earth metal magnesium is 1.74g/cm3, which is about 2/3 of the density of metal aluminum. Magnesium-lithium alloy made of magnesium and lithium, when the lithium content reaches 20%, its density is only 1.2g/cm3, becoming the lightest alloy; when the lithium content exceeds 5%, the β phase can be precipitated to form (α +β) Two-phase coexistence structure; when the lithium content exceeds 11%, the magnesium-lithium alloy becomes a single phase, thus improving the plastic workability of the magnesium-lithium alloy. Adding the third element (such as Al, Cu, Zn, Ag or Ce, La, Nd, Y and other rare earth elements) to the magnesium-lithium alloy not only refines the alloy structure, but also greatly improves the tensile strength and elongation at room temperature Rate, and high temperature plasticity appears under certain deformation conditions.
Lithium metal is a catalyst and intermediate for the synthesis of pharmaceuticals, such as the synthesis of vitamin A, vitamin B, vitamin D, adrenal cortex hormones, and antihistamines. Lithium compounds are often used clinically, such as lithium carbonate, lithium acetate, lithium tartrate, lithium oxalate, lithium citrate, lithium bromide, lithium iodide, lithium naphthenate, and lithium urate, among which lithium carbonate is the main one. Because lithium carbonate is stable under general conditions, easy to store, and easy to prepare, the lithium content in it is relatively high, and its oral absorption is quick and complete. As mentioned above, the preparation of a compound lithium salt supplemented with an antidepressant has an obvious effect on manic depression.
Some chemical reactions of lithium are as follows.
(1) The reaction between lithium and air. Lithium metal can be easily cut with a knife. You can see a shiny silvery surface, but it will quickly become gray due to its reaction with oxygen and water vapor in the air. When lithium is ignited in the air, the main product is Li2O, an oxide of white lithium. Certain lithium peroxides, Li2O2, are also white.
4Li(s)+O2(g)→2Li2O (s)
2Li(s)+O2(g)→Li2O2(s)
(2) Lithium and water reaction Lithium metal can slowly react with water to produce colorless lithium hydroxide solution (LiOH) and hydrogen (H2). The resulting solution is alkaline. Because of the formation of hydroxide, the reaction is exothermic. As mentioned earlier, the reaction speed is slower than the reaction of sodium and water.
2Li(s)+2H2O→2LiOH(aq)+H2(g)
(3) Lithium and halogen reaction Lithium metal can react with all halogens to form lithium halide. Therefore, it can react with F2, Cl2, Br2, and I2 to sequentially generate monovalent lithium fluoride (LiF), lithium chloride (LiCl), lithium bromide (LiBr) and lithium iodide (LiI). The reaction formula is as follows.
2 Li(s)+F2(g)→2LiF (s)
2Li(s)+Cl2(g)→2LiCl(s)
2Li(s)+Br2(g)→2LiBr(s)
2Li(s)+I2(g)→2LiI(s)
(4) Lithium and acid reaction Lithium metal is easily soluble in dilute sulfuric acid, and the resulting solution contains water and hydrated monovalent lithium ions, sulfate ions and hydrogen gas, such as reacting with sulfuric acid.
2Li(s)+H2SO4(aq)→2Li+(aq)+SO42﹣(aq)+H2(g)
(5) Lithium reacts with alkali. Lithium metal reacts slowly with water to form a colorless lithium hydroxide solution and hydrogen.
(H2). The reaction will continue when the solution becomes alkaline. As the reaction progresses, the hydroxide concentration increases.
Lithium is the lightest metal with high electrode potential and high electrochemical equivalent, and its electrochemical specific energy density is also quite high. These unique physical and chemical properties of lithium determine its important role. Lithium compounds have remarkable performance as cathode materials for high-energy batteries, such as lithium secondary batteries used for charging, such as lithium MnO2, lithium Mn2O4, and lithium CoO2 battery cathode materials. This type of battery has long life, high power, high energy, and can be used at low temperatures. It has been applied to ballistic missiles in national defense and will be used in civil fields such as electric vehicles; LiCl-KCl system and aluminum-lithium alloy-FeS system are also Used as a large-capacity battery for the production of electrolyte; lithium hydroxide is used as an electrolyte potassium hydroxide additive for alkaline batteries such as Ni-Cd.
In the early 1990s, Sony Energy Development Corporation of Japan and Moli Energy Corporation of Canada respectively developed or worked on a new type of lithium-ion battery, which not only has good performance, but also has no pollution to the environment. With the rapid development of information technology, portable machinery and electric vehicles, the demand for high-efficiency power supplies has increased dramatically, and lithium batteries have become one of the fastest-growing fields in the past. Since the specific energy density and specific power density of lithium ion batteries are more than 4 times that of nickel separator batteries, in recent years, lithium ion secondary batteries have developed rapidly at an average annual rate of 20%. The new polymer lithium-ion battery recently developed in the United States has the characteristics of small size, safety and reliability, and its price is only 1/5 of that of lithium-ion batteries. At present, a high-energy density secondary lithium battery with a weight specific energy density of 180W·h/kg, a volume specific energy of 360W·h/L, and more than 500 charge and discharge cycles is being developed, which will be used in electric vehicles. It is estimated that in the first ten years of this century, lithium carbonate used in lithium batteries will exceed 20,000 tons. At the same time, the molten carbonate fuel cell with lithium salt as the electrolyte is expected to become the second-generation fuel cell after the phosphate fuel cell, and its development is eye-catching.

















