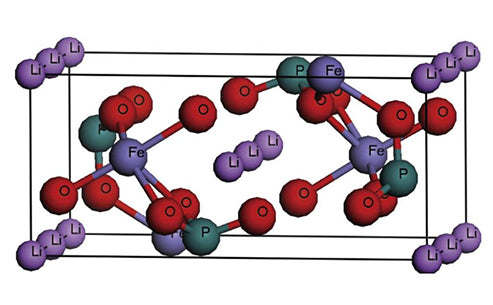
①Olivine phase
In 1997, the advent of olivine crystal type materials, especially after the discovery of LiFePO4 compounds, LiFePO4 has become one of the most challenging cathode materials with low cost, multiple elements, and at the same time being environmentally friendly. It plays an important role in the electrochemical energy storage of lithium ions. The discharge voltage of the battery assembled by it reached 3.4V, and there was no obvious capacity decay after hundreds of cycles. In addition, the capacity of the battery reached 170mA·h/g, and its performance was better than LiCoO2 and LiNiO2. Moreover, the material has good stability in the charge and discharge test.
LiFePO4 can be synthesized by a high-temperature synthesis method or a sol-gel method under the condition of an aqueous solution. However, although olivine can be easily synthesized in just a few minutes in an aqueous environment, its electrochemical performance is relatively poor. Structural analysis shows that nearly 7% of the iron atoms are concentrated around the lithium ion, which affects the lattice parameters of the crystal to a certain extent. Compared with the ordered arrangement of LiFePO4, a=1.0333nm, b=0.6011mc=0.4696nm , The parameters of the synthesized olivine phase material are a=1.0381nm, b=0.6013nm, and c=0.4716nm, respectively, and the iron atoms therein substantially hinder the mass transfer and diffusion of lithium ions. Therefore, it is very important to take relevant measures to make the lithium ions and iron atoms in the material arranged in an orderly manner. Heating the above materials to 700°C under hydrothermal conditions can solve the problem of disordered arrangement of ions. Recent studies have found that optimization of synthesis conditions can further improve the properties of materials prepared by hydrothermal synthesis. For example, after adding an inert film of ascorbic acid that can prevent the formation of iron on the surface of the material, the material can exhibit excellent electrochemical performance even without a carbon coating layer.
The conductivity of the above-mentioned materials is relatively low at room temperature, so its theoretical capacity can only be reached under conditions of very small current density or increased temperature. The reason is that the diffusion rate of lithium ions on the interface is too slow. The study found that the carbon coating can significantly improve the electrochemical performance of the material. Sucrose is used as a carbon precursor and used in hydrothermally synthesized samples. The conductivity of the pure LiFePO4 sample is 10-9S/cm, while the conductivity of the sample made of the material with the layered carbon structure reagent can reach 10-5~10-6S/cm. When the material is coated with carbon gel in the synthesis stage, if a lower carbon content of 5mg/cm² is used, its capacity can reach 100%, and only 20% when the carbon content is higher. At a higher cycle rate, 800 cycles can be obtained, and the electrochemical capacity of the material is maintained at 120mA·h/g. After the carbon-containing material is fully ground, its capacity can be increased even under high temperature conditions. LiFePO4 can also show good electrochemical performance after doping with niobium and other elements. What causes the electrical conductivity of the material to change? It may be that the increase in electrical conductivity is related to the formation of a Fe2P thin film layer, which is a highly conductive material in the material. The thin film can be formed in a high temperature environment, especially in the presence of a reducing agent such as carbon.
The structure of olivine is shown in Figure 1. It can be seen that FePO4 is formed on the lithium ion migration surface. In fact, FePO4 and LiFePO4 have the same structure, and this FePO4 and another compound Fe0.65Mn0.35 PO4 are also crystals of the same structure. From the cycle curve of Figure 2(a), it can be seen that there is a two-phase system in the equilibrium state of LiFePO4 and FePO4.
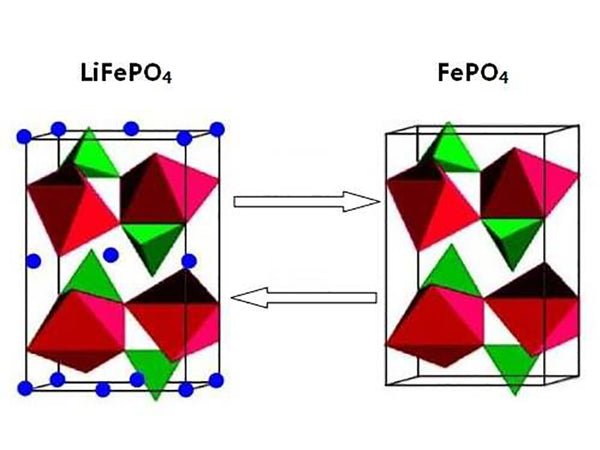
Figure 1 Schematic diagram of orthogonal LiFePO4 and quartz-like triangular pyramidal FePO4
It can be seen from Figure 2 that when the test control temperature is 60℃ and the current density is 1mA/cm², even in the electrode containing 80mg/cm² of active material, the battery's material utilization during the cycle can still reach 100%; at room temperature , When the current density is 1mA/cm² and 0.1mA/cm², it can reach 70% and 100% respectively; even when the current density is 10mA/cm² and the packing density is low, it can still reach 70%. As shown in Figure 2(b).
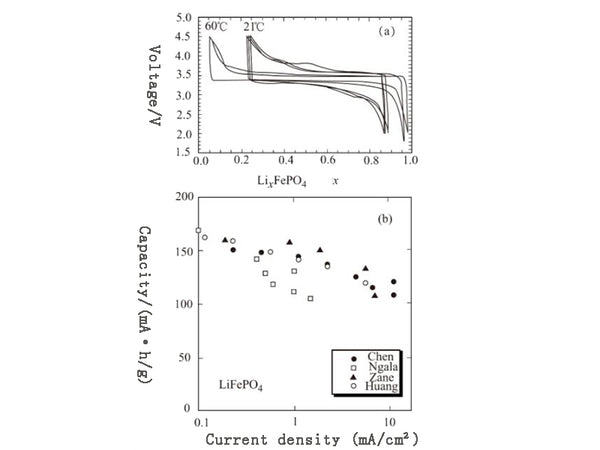
Figure 2 Electrochemical performance of LiFePO4
The electrochemical performance of LiFePO4 mainly depends on its chemical reaction, thermal stability and the product LiFePO4 after discharge. In the test of assembled battery or the experiment of differential calorimetry scanning, no thermal deviation was found in the range of 25~85℃. However, this olivine structure is unstable, because its spatial position is at the edge of the octahedral crystal and tetrahedral crystal structure, and it is easily converted into a spinel type under the action of external pressure. The conversion of orthorhombic crystal system to olivine type in LiFePO4 lattice has also been reported. The electrochemical activity of this configuration material is inactive. In addition, it is known that FePO4 has more than two crystal arrangements: one is the orthorhombic arrangement of the same structure as LiFePO4, and its ions exist in the octahedral structure of FePO6; the other is the triangular crystal system, and its ions Exist in the tetrahedral structure of FePO4. The focus of the research is the octahedral crystal structure. The triangular arrangement is composed of FePO4 and PO4. Each FePO4 tetrahedron is connected to four PO4 tetrahedrons through its four corners and has crystal defects, resulting in a structure similar to quartz. However, the electrochemical reaction activity of all tetrahedral arrangements is not high, mainly because the Fe2+ in the tetrahedral structure is unstable.
The stability of the battery and its electrode materials is related to the length of the service life, so it is very important to better understand the orthorhombic crystal structure of LiFePO4 and FePO4. For example, what happens when LiFePO4 is in an over-discharged state? Will the orthorhombic FePO4 slowly transform into a quartz structure? There are reports in the literature that when iron phosphate reacts with excess n-butyllithium to form lithium phosphate and iron compounds, refer to Table 1. Obviously, when the discharge potential is very low, the phosphate spatial lattice structure is easily damaged. Compared with pure lithium, the potential of n-butyl lithium ion is about 1V. Using LiFePO4 as the cathode material, the discharge voltage dropped to 1.0V at a current density of 0.4mA/cm², confirming that the lithium ions reacted with the damaged LiFePO4 and caused the loss of capacity. After 5 cycles, it is attenuated by 80%. Therefore, if lithium phosphate batteries are to be used in commercial applications, they also need overcharge protection. From another perspective, there is no evidence to show that the metastable orthorhombic structure of FePO4 can be transformed into a quartz triangle structure under conventional electrochemical conditions. More importantly, there is no tendency to lose oxygen during the migration of the spatial lattice of lithium ions.
Due to the low viscosity of LiFePO4, the bulk density of LiFePO4 is relatively low. Therefore, it is necessary to add small-volume, high-density carbon and organic coupling agent to the electrode material. The densities of LiFePO4, Teflon, and carbon black are 3.6g/cm3, 2.2g/cm3, 1.8g/cm3, respectively. If 10% carbon and 5% Teflon are added to the electrode, 25% less than the theoretical value can be obtained. Energy density per unit volume. This indicates that all particles in the electrode structure can be arranged in a balanced and effective manner. After being combined with carbon, especially the decomposed cellulose, there will be no messy structure. Studies have reported that the above-mentioned carbon atoms are arranged in disorder, so the reduction of unit volume density is very important. In order to optimize the electrochemical performance of the electrode, different types of carbon have been used in this area of research to find the optimal addition amount of carbon. When the content is 6%, the polarization of the electrode is slightly higher, and basically no difference is observed in the others. However, whether it is carbon black, carbon gel, glucose, or hydrogel, the method of adding carbon does not seem to be very good. The calcination temperature of the reaction mixture seems to be more effective, because calcination can determine the amount of graphite carbon compound on the surface of LiFePO4. Experiments have found that the sp2 hybrid carbon is more effective than the sp3 structure. In addition, the carbon in the reaction material can basically control the size of the structured particles. At the same time, the reaction temperature has a great influence on the performance of the material, and the best temperature is 650~700℃.
The above discussion of LiMPO4 olivine structure series materials mainly focused on the doping of iron. In fact, the available transition metals also include manganese, nickel, cobalt and so on. Their discharge voltage is relatively high, but the electrochemical performance of these doped element materials is worse than that of iron-doped compounds. The discharge curve platform of the iron-manganese composite is different from that of the iron and manganese oxides alone. Due to the Jahn-teller effect of Mn3+, LiMPO4 cannot release lithium, making it thermodynamically more unstable olivine-type MnPO4; LiMPO4 prepared by the direct precipitation method is studied, and the material is ball-milled with carbon black to coat the electrode with carbon Later, the available capacity is 70mA·h/g, and it is also reversible. The above shows that the thermodynamic properties of MnPO4 are stable. Research on LiMnPO4, a thermal decomposition product of LiMnPO4(OH), found that the electrode potential of Mn2+/Mn3+ changes around 4.1V, the electrode capacity is very low and a high polarization peak appears. After adding carbon black and heating, the capacity of the electrode is not significantly improved. Using theoretical methods to calculate the open circuit voltage and the energy band gap in phosphate, it is found that they are both relatively high. The open circuit voltage of LiFePO4 is 3.5V, LiMnPO4 is 4.1V, LiCoPO4 is 4.8V, and LiNiPO4 is 5.1V. This can explain why The electrochemical activity of LiNiPO4 is not high in the conventional cycle potential range. The calculated values of LiFePO4 and LiMnPO4 are 3.7 eV, and the band gap of 3.8 eV coincides with their color and diffuse reflectance spectrum. This also reveals that different band gaps cannot explain the electrochemical behavior of different electrode materials. Electronic conductivity is likely to be related to the polarization mechanism.
Figure 3 is an SEM image of LFePO4 sintered at 600°C under inert atmosphere conditions.
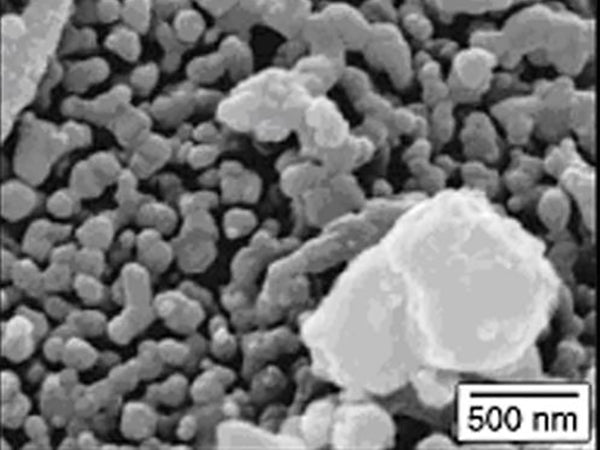
Figure 3 SEM image of LiFePO4 sintered at 600℃ under inert atmosphere
②Phosphate phase of other Fe
The structure of several other iron phosphate salts, such as Li3Fe2 (PO4)3, has a discharge voltage lower than 3V. Compared with LiFePO4, the material has a lower value for use. Using the hydrothermal method, the electrochemical performance of FePO4 (nH2O) with crystal water and anhydrous phases was studied, and the amorphous and crystalline structure of FePO4 (nH2O) were obtained by heating and drying in different temperature ranges. It is found that the electrochemical capacity of the material made of FePO4 (nH2O) with amorphous structure is more than 2 times higher than that made with crystal structure. This may be related to the amorphous structure of the former and the crystalline structure of the latter. Whether it is an amorphous structure or a crystalline material, the chemical behavior of LixFePO4 is the main reaction, while the LiFePO4-FePO4 system is a two-phase reaction. In a different temperature range, the current density is 0.2mA/cm², and the electrode potential is from 2-4V. The discharge curve of amorphous structure FePO4.
Another type of iron phosphate is mainly derived from ore raw materials called Giniit and Lipscombite. Its structure is composed of coplanar FeO6 octahedrons stacked orthogonally to each other, so it can provide a channel for lithium ion diffusion. However, the discharge platform of the above materials is slightly inclined, and the voltage value is lower than that of the LiFePO4-FePO4 system.