
The use of electrolyte additives to achieve battery overcharge protection is of great significance for simplifying battery manufacturing processes and reducing battery costs. The realization of the electrolyte's overcharge protection function for the battery through electrolyte additives can be considered from the following aspects.
Overcharge protection additive
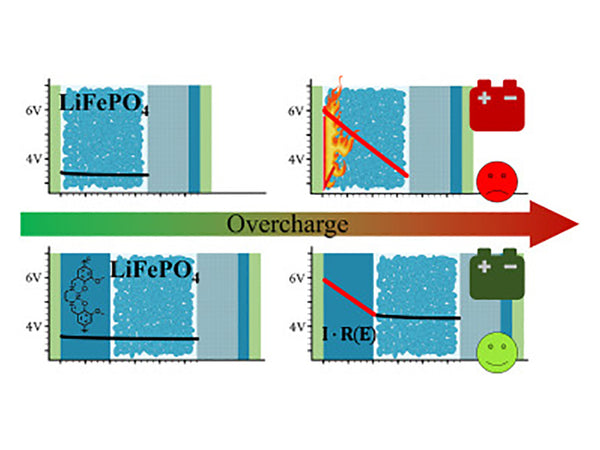
① Redox shuttle. By adding an appropriate redox pair to the electrolyte, the battery is internally protected. The principle of the redox shuttle is: during normal charging, the added redox pair does not participate in any chemical or electrochemical reactions. When the charging voltage exceeds the normal charging cut-off voltage of the battery, the additive begins to oxidize at the positive electrode, and the oxidation product diffuses to the negative electrode to cause a reduction reaction, as shown in equations.
Positive electrode: R→O+ne
Negative electrode: O+ne→R

Redox Shuttle Additives
After the battery is charged, the redox couple shuttles between the positive electrode and the negative electrode, absorbing the excess charge, forming an internal anti-overcharge mechanism, and greatly improving the safety performance and cycle performance of the battery. Therefore, this additive is vividly called "redox shuttle" or "internal chemical shuttle".
In the LiAsF6/THF electrolyte, the I2 formed by the oxidation of LiI during overcharge will trigger the ring-opening polymerization of THF. In order to avoid the occurrence of the above reaction, an excessive amount of LiI must be added to the organic electrolyte to form a stable LiI3 with I2. In addition, Li+ will react with I2 to form LiI, which reduces the stability of the passivation film on the lithium surface and accelerates the dissolution of lithium. Therefore, the effect of using this redox shuttle on battery safety is not obvious. Ferrocene and its derivatives can also be used as redox couples to prevent battery overcharging, but the redox potentials of these compounds are mostly between 3.0 and 3.5V, which is not suitable for use in lithium ion batteries. The oxidation potential of the complex of 2,2-pyridine and 1,10-phenanthroline of ferrous ion is about 0.7V higher than the oxidation potential of ferrocene, which is near V. The redox potentials of the ortho- and para-dimethoxy-substituted benzenes are above 4.2V and can undergo reversible redox reactions, as shown in Figure 1, so they can be used as additives to prevent overcharge.
Some aromatic compounds with thidium derivatives with acetyl or other functional groups are stable up to 4.2~4.3V. Using them as a chemical overcharge protection agent can consume excess current when the battery is overcharged. When they are introduced into C/LiCoO2 prismatic batteries as electrolyte additives, they will be oxidized above 4V and become oxidized shuttles. Using ARC (accelerating rate calorimeter, acceleration rate calorimeter) to study the thermal properties of batteries containing the above materials proved that the current provided to the charging process is not stored, but is quickly and thoroughly consumed in the oxidation-reduction reaction. In the commonly used electrolyte solution 1mol/LPC/LiClO4, 10 kinds of organic aromatic compounds including biphenyl, cyclohexylbenzene (eyclohexylbenzene) and hydrogenated diphenyleneoxide (hydrogented diphenyleneoxide) were added respectively. Cyclohexylbenzene and Hydrogenated dibenzofuran additives have better overcharge resistance and higher cycle efficiency than biphenyl. On the cyclic voltammetry curve, the starting potential of the oxidation reaction in the second week and the third week of the battery using these additives is lower than the starting potential of the oxidation reaction in the first week. This behavior indicates that the product of the oxidation reaction is more prone to oxidation than the original aromatic compound.
When the battery is overcharged, the additives are electropolymerized to form a conductive polymer film, which short-circuits the positive and negative electrodes and prevents the battery from being charged to a higher voltage. Use biphenyl as an anti-overcharge additive. When the charging voltage reaches 4.5-4.7V (relative to Li/Li+), the added biphenyl undergoes electrochemical polymerization, forming a conductive film on the surface of the positive electrode. The deposited film can penetrate the separator to reach the surface of the negative electrode, causing an internal short circuit of the battery and preventing the battery voltage from running out of control. On the other hand, the electro-oxidative polymerization of biphenyl generates excessive gas and heat, which helps to improve the sensitivity of the electrical open circuit device.
When the charging voltage reaches a certain threshold, the electrolyte additives undergo electropolymerization, forming a polymer film that is double-insulating for electrons and ions, preventing charge exchange between the positive and negative electrodes through the internal circuit, making the charging process unable to continue. For example, xylene is selected as the overcharge protection agent for lithium-ion batteries, and the overcharge curve, cyclic voltammetry behavior, and SEM observations of batteries using xylene additives have found that this type of additives polymerizes on the surface of the positive electrode when overcharged. The dense insulating polymer film prevents further oxidation of electroactive materials and electrolytes, and improves the overcharge resistance of lithium-ion batteries.
② Flame-retardant electrolyte. Adding some high boiling point, high flash point and non-flammable solvents to the battery can improve the safety of the battery. Fluorinated organic solvents have the characteristics of high flash point and non-flammability. Adding them to the organic electrolyte helps to improve the safety performance of the battery under heating, overcharge and discharge conditions. Some fluorinated chain ethers, such as C4F9OCH3, are used to improve the safety performance of lithium ion batteries, but the dielectric constant of fluorinated chain ethers is generally low, and the solubility of electrolyte lithium salt in them is very small. Fluorinated chain ethers It is also difficult to be miscible with other organic solvents with high dielectric constants such as EC and PC. Fluorinated cyclic carbonate compounds such as monofluoromethyl ethylene carbonate (CH2F-EC), difluoromethyl ethylene carbonate (CHF2-EC), trifluoromethyl ethylene carbonate (CF3-EC), etc. It has good chemical stability, high flash point and dielectric constant, and can dissolve electrolyte lithium salt well and is miscible with other organic solvents. Batteries using these additives have good charge and discharge performance and cycle performance.
Flame retardant additives mainly use some phosphorus-containing compounds. Add a certain amount of flame retardant such as organophosphorus series, silicon boron series and boric acid ester series to the organic electrolyte. For example, after adding flame retardant [NP(OCH3)2]3, the heat generation rate of the battery is significantly reduced, and the battery capacity is also significantly improved; such as 3 phenyl phosphonate (TPP) and 3-butyl phosphonate (TBP) can be used as a flame retardant for lithium-ion battery electrolyte. Fluorinated phosphates and alkyl phosphates have flame retardant effects and do not reduce other performances of lithium-ion batteries. Hexamethylphosphoryl (HMPA) can be used as a flame retardant for lithium-ion battery electrolytes. The flammability, electrochemical stability, electrical conductivity of HMPA and the cycling performance of electrolytes containing HMPA were studied. The addition of HMPA compound to the electrolyte solution containing LiPF6 and organic carbonate significantly reduces the flammability of the electrolyte, but the addition of HMPA caused a slight decrease in electrolyte conductivity and narrowed the electrochemical stability window. The battery cycle after using flame retardant additives Performance is reduced.
③Self-closing electrolyte additive. The thermal shutdown mechanism is set in the electrolyte of the battery, which is somewhat similar to the thermal shutdown mechanism of the polymer separator. The PVdF-HFP/PE composite gel electrolyte with thermal shutdown function can improve the safety of lithium-ion batteries as the internal safety device of the battery. The composite gel electrolyte includes PVdF-HFP polymer, polyethylene (PE) thermoplastic resin and 1.0mol/L LiClO4/PC/EC (or LiPF6/γ-BL+EC) plasticizer. When the content of PE exceeds 23% (mass fraction), the resistance of the composite gel electrolyte increases rapidly by several orders of magnitude. Near the melting point of PE (90°C or 104~115°C), SEM observation shows that the melting point of PE is The nearby PE particles dispersed uniformly in the PVdF-HFP gel electrolyte fuse into a continuous film. The continuous PE film can cut off the diffusion channel of ions between the positive and negative electrodes to prevent thermal runaway of the battery.

PVdF-HFP/PE composite gel electrolyte
















