What is Lithium ion battery electrolyte?
The electrolyte is one of the main components of the battery, and its function has nothing to do with the battery device. Electrolytes are used in batteries, capacitors, and fuel cell devices to transport ions between the positive and negative electrodes through the battery. It has an important influence on battery capacity, operating temperature range, cycle performance and safety performance. Because its physical location is in the middle of the positive and negative electrodes and is closely related to the two electrodes, when a new electrode material is developed, the development of the supporting electrolyte must also be carried out simultaneously. In a battery, the chemical properties of the positive and negative materials determine the output energy. For the electrolyte, in most cases, the mass flow ratio in the battery is controlled to control the energy release rate of the battery.
According to the morphological characteristics of the electrolyte, the electrolyte can be divided into two categories: liquid and solid, both of which are substances with high ionic conductivity, which play a role in transferring the charge between the positive and negative electrodes inside the battery. Different types of batteries use different electrolytes. For example, the electrolytes of lead-acid batteries use aqueous solutions; as the electrolyte of lithium-ion batteries, aqueous solutions cannot be used. This is because the water has a small hydrogen and oxygen evolution voltage window, which cannot meet the high requirements of lithium-ion batteries. Voltage requirements; in addition, the current used lithium-ion battery cathode materials have poor stability in water systems.
Therefore, the electrolytes of lithium ion battery all use organic solutions of lithium salts as electrolytes (such as LiPF6/EC+DMC). However, due to the convenient source of the aqueous solution system and its higher conductivity, researchers are also working hard to develop new electrolytes in this area.
(1) Non-aqueous system electrolyte
In the manufacturing process of lithium ion battery, the general principles for selecting electrolyte are as follows:
①Good chemical and electrochemical stability, that is, it basically does not react with the electrode materials of the battery system, such as the positive electrode, the negative electrode, the current collector, and the separator binder;
②High ionic conductivity, generally 1×10-3~2×10-2S/cm, high dielectric constant, low viscosity, and low resistance to ion migration;
③High boiling point and low freezing point, keep liquid in a wide temperature range, the general temperature range is -40~70℃, suitable for improving the high and low temperature characteristics of the battery;
④The solubility of the added solute is large;
⑤Higher cycle efficiency for battery positive and negative poles;
⑥ It has good comprehensive physical and chemical properties, such as low vapor pressure, good chemical stability, non-toxic and non-flammable.
In addition to the above requirements, the electrolyte used for lithium ion battery should generally meet the following basic requirements:
①High thermal stability, no decomposition in a wide temperature range;
②Wide electrochemical window, keep the stability of electrochemical performance in a wide voltage range;
③It has good compatibility with other parts of the battery, such as electrode materials, electrode current collectors and separators;
④Any component of the electrolyte is easy to prepare or purchase;
⑤ It can best promote the reversible reaction of the electrode.
Electrolyte lithium salt is the source of lithium ions. A suitable electrolyte lithium salt should have the following conditions: good thermal stability, not easy to decompose; high ion conductivity in the solution; good chemical stability, that is, it will not react with solvents and electrode materials. Reaction; good electrochemical stability, the oxidation potential of the anion is high and the reduction potential is low, and it has a wide electrochemical window; the molecular weight is low, and it has good solubility in a suitable solvent; it can make lithium in the positive and negative electrode materials The amount of embedding is high and the reversibility is good; the cost of the electrolyte is low.
Commonly used lithium salts are LiClO4, LiBF6, LiPF6, LiAsF6 and some organic lithium salts, such as LiCF3SO3, LiC (SO2CF3) 3 and so on. In the process of preparing the electrolyte, the above-mentioned lithium salt is dissolved in the solvent system in FIG. 1 according to a certain proportion to form the electrolyte for lithium ion battery. The commonly used electrolyte systems for lithium-ion batteries are 1mol·L-1LiPF6/PC-DEC (1:1), PC-DMC (1:1) and PC-MEC (1:1) or 1mol·L-1LiPF6/EC- DEC (1:1), EC-DMC (1:1) and EC-EMC (1:1).
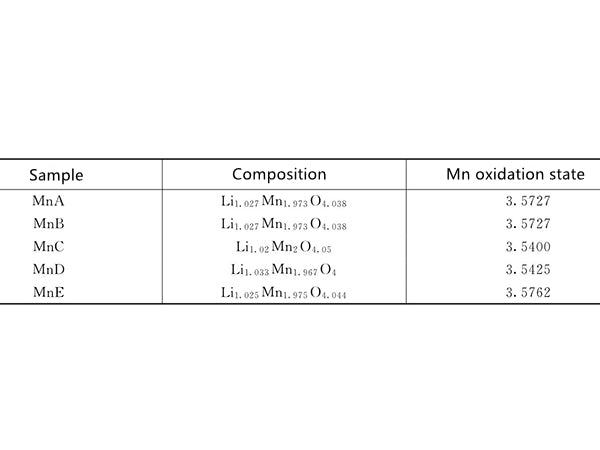
figure 1 Composition of LiMn2O4 samples with different composition and oxidation state of Mn
Since the ionic conductivity of the electrolyte determines the internal resistance of the battery and the electrochemical behavior at different charge and discharge rates, it is very important to the electrochemical performance and application of the battery. Generally speaking, the conductivity of aprotic organic solvents dissolved with lithium salts can reach up to 2×10-2S/cm, but it is much lower than that of aqueous electrolytes. Many lithium-ion batteries use mixed-solvent system electrolytes, which can overcome some of the disadvantages of a single-solvent system. The electrolyte formulation illustrates this point. When the electrolyte concentration is high, its conductive behavior can be explained by the ion pair model.
In addition to the electrochemical performance of the electrolyte that is affected by the conductivity of the electrolyte, the electrochemical window of the electrolyte and its reaction with the battery electrodes are also critical to the performance of the battery.
The so-called electrochemical window refers to the difference between the potential Eox for oxidation and the potential Ered for reduction. As a battery electrolyte, the first necessary condition is that it does not react with the negative electrode and the positive electrode material. Therefore, Ered should be lower than the oxidation potential of metal lithium, and Eox should be higher than the lithium insertion potential of the positive electrode material, that is, oxidation (positive) and reduction (negative) reactions must not occur in a wide potential range.
Generally speaking, the oxidation potential of ether compounds is lower than that of carbonates. Solvent DME is generally used for primary batteries. The oxidation potential of secondary batteries is relatively low. Common 4V lithium-ion batteries must compensate for overpotentials during charging. Therefore, the electrochemical window of the electrolyte is required to reach about 5V.
In addition, the measured electrochemical window is related to the working electrode and current density. The electrochemical window is also related to organic solvents and lithium salts (mainly anions). The order of oxidation reaction potential of some solvents is: DME(5.1V)<THF(5.2V)<EC(6.2V)<AN(6.3V)<MA(6.4V)<PC(6.6V)<DMC(6.7 V), DEC (6.7V), EMC (6.7V). For organic anions, the oxidation stability is related to the substituents. The introduction of electron withdrawing groups, such as F and CF3, is conducive to the dispersion of negative charges and improves its stability. Using glassy carbon as the working electrode, the order of the oxidation stability of anions is: BPh4-<ClO4-<CF3SO3-<[N(SO2CF3)2]-<C(SO2CF3)3-<SO2C4F9-<BF4-<AsF6-< SbF6-.
The reaction between the electrolyte and the electrode is mainly for the reaction with the negative electrode, such as graphitized carbon. From a thermodynamic point of view, because organic solvents contain type groups, such as C-O and C-N, the negative electrode material reacts with the electrolyte. For example, using noble metal as the working electrode, PC will be reduced when the voltage is lower than 1.5V (using lithium metal as the reference) to produce lithium alkyl carbonate. Since a protective film is formed on the surface of the negative electrode that can pass lithium ions, it prevents further reduction of the negative electrode material and the electrolyte, so it is kinetically stable. If a mixed solvent of EMC and EC is used, the performance of the protective film will be further improved.
For carbon materials, the structure is different, the electrochemical behavior of the same electrolyte component is also different; for the same carbon material, the electrochemical behavior of different electrolyte components is different. . For example, for synthetic graphite, in the 1mol/L Li[N(SO2CF3)2] solution of PC/EC, the irreversible capacity of the first cycle is 1087mA·h/g, while in EC/DEC 1mol/L Li[ The first irreversible capacity in N(SO2CF3)2] solution is only 108mA·h/g. It reacts with water to generate LiOH, etc., which may lose the performance of the protective film, causing the continued reduction of the electrolyte. Therefore, in the organic electrolyte, the moisture content is generally controlled below 20×10-6.
The partial reaction of solvent and impurities on the carbon negative electrode has the following reaction formula:
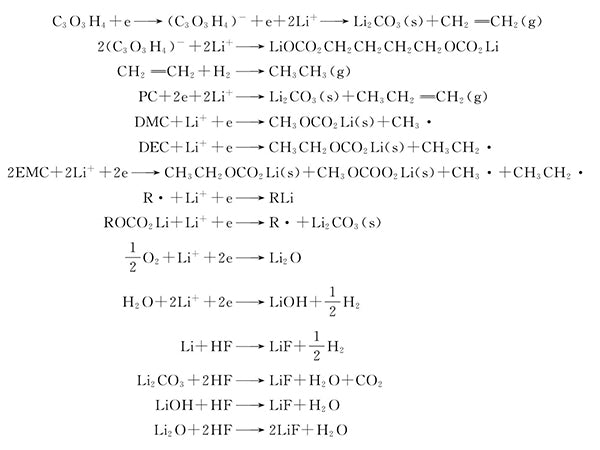
The reaction between the electrolyte and the cathode material is mainly based on the oxidizing property of the electrolyte. Figure 2 shows the oxidative decomposition potential of acrylic carbonate-based electrolyte with the type of salt and the change of electrode material. It provides important information and data for designing the electrolyte system of the battery.
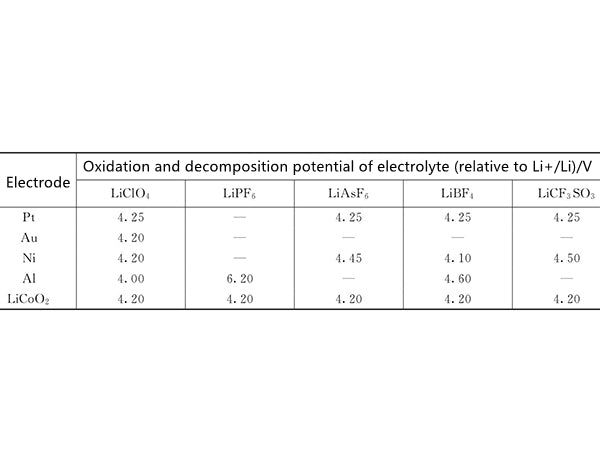
figure 2 The influence of different salts and electrode materials on the oxidative decomposition potential of acrylic carbonate-based electrolyte
The electrolyte lithium salt is relatively active, and the reduction reaction occurs preferentially, and it is the main component of the interface protective film. The step-by-step reactions that occurred are as follows:
Li [N(SO2CF3)2] +ne+nLi+---->Li3N+Li2S2O4+LiF+ LiyC2Fx
Li [N(SO2CF3)2] +2e+2Li+---->Li2NSO2CF3+LiSO2CF3
Li2S2O4+4e+4Li---->Li2SO3+Li2S+Li2O
(2) Solid electrolyte
The use of polymer electrolytes for lithium-ion batteries has reached the level of commercialization. Polymer electrolytes can be divided into pure polymer electrolytes and colloidal polymer electrolytes.
Pure polymer electrolytes are difficult to commercialize due to their low room temperature conductivity. The colloidal polymer electrolyte uses liquid electrolyte molecules fixed in a polymer network with a suitable microstructure to achieve its ionic conduction. This type of electrolyte has the stability of a solid polymer and the high ionic conductivity of a liquid electrolyte. Good application prospects.
The colloidal polymer electrolyte can be used as the electrolyte of the lithium ion battery, but also can act as a separator, but due to its poor mechanical properties, complicated preparation process or poor conductivity at room temperature, and the colloidal polymer electrolyte is essentially a thermodynamically unstable system , In an open environment or when stored for a long time, the solvent will ooze out of the surface, resulting in a decrease in its conductivity. Therefore, the colloidal polymer electrolyte completely replaces the polyethylene and polypropylene separators and serves as the separator of the lithium ion battery alone. There are still many problems to be solved.
The electrolyte in the battery system is an ion carrier (it must be an insulator for electrons). The polymer electrolyte used in lithium ion batteries meets the requirements of the liquid electrolyte of lithium ion batteries, such as chemical stability and electrochemical stability, etc. , Should also meet the following requirements: polymer film has excellent processability; high room temperature conductivity, high lithium ion conductivity at low temperature; good high temperature stability, not easy to burn; good bending performance, good mechanical strength.
















