
Facing the energy density anxiety and safety issues of traditional liquid lithium-ion batteries, all solid state battery (ASSB) using sulfide solid-state electrolytes (SSEs) with high ionic conductivity are expected to simultaneously solve the sulfide all-solid-state-battery safety hazards and achieve high energy/power density. To obtain sulfide all-solid-state battery with high energy density, layered oxide NCM cathode materials are widely used in sulfide-based ASSBs.
However, studies have shown serious interfacial chemical mismatch and chemical instability between NCMs and sulfide SSEs. Especially at high voltage, the interface problem will be more complicated. This article will provide a detailed introduction and specific analysis of the latest breakthroughs in sulfide all-solid-state battery technology to help you gain a comprehensive and in-depth understanding.
1.The latest research results
Recently, a research group from a research institute in China successfully synthesized a surface-coated TiNb2O7 (TNO) and bulk-doped Ti-coated-doped ternary material (DC-TNO@ SCNCM). For the first time, a coating-doping synergistic modification strategy for high-voltage SCNCM cathode materials was proposed in sulfide all-solid-state battery. The results show that the decomposition of sulfide SSEs can be avoided by a uniform TNO coating with thermodynamic/electrochemical stability and electronic insulation.
Meanwhile, the formation of strong Ti-O bonds in the SCNCM doped Ti can effectively stabilize the lattice oxygen and prevent the generation of oxygen-containing by-products at the interface. Coating-doping can realize the synergistic modification of the SCNCM cathode material from the surface to the bulk phase, which fundamentally stabilizes the interface and reduces the interface resistance. Based on this, the modified DC-TNO@SCNCM cathode material can be stably cycled for 140 cycles at a high cut-off voltage of 4.4 V, and the capacity retention rate is as high as 92.2%.
2.Presentation of research content
High-nickel single crystal ternary LiNi0.6Co0.2Mn0.2O2 (SCNCM) is used as a cathode material, which has no grain boundaries inside the particles, and has inherent integrity and a continuous conductive network. Better mechanical strength can suppress the cracking problem of the particles. Among the coating materials, the research on LiNbO3 and Li4Ti5O12 is the earliest and most extensive. The structure and properties of TiNb2O7 with two-dimensional ion diffusion channels are similar to Li4Ti5O12 and LiNbO3. Theoretical studies also show that Ti and Nb oxide coatings exhibit great stability in contact with high-voltage oxide cathodes.
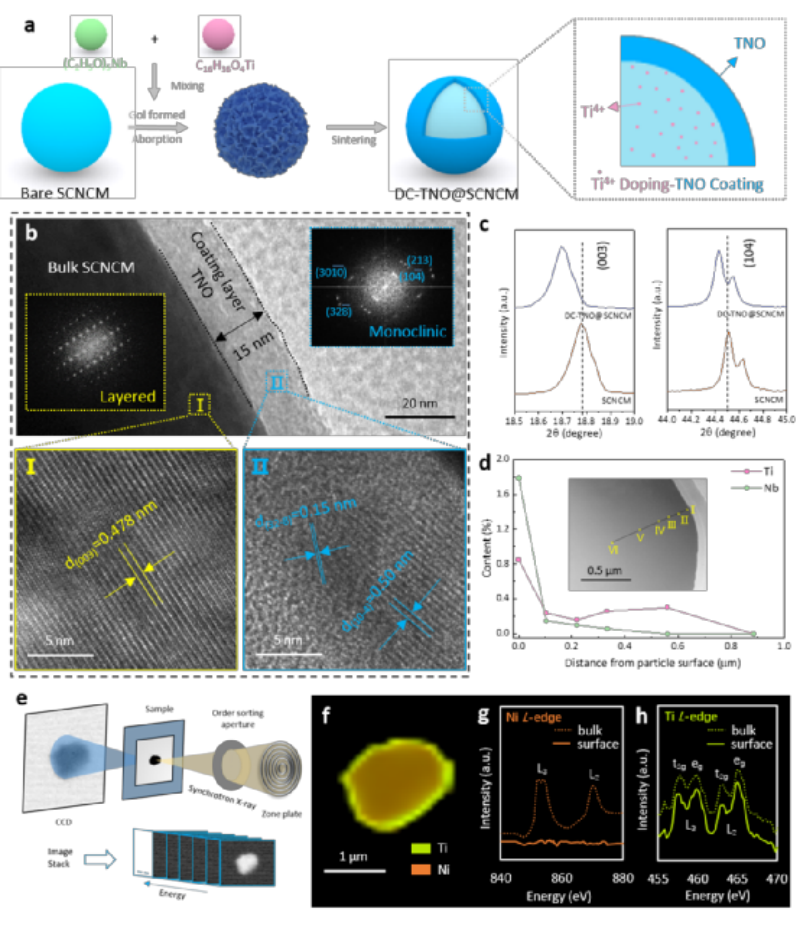
Fig. 1 Characterization of DC-TNO@SCNCM. a) Schematic diagram of the synthetic process of doping-coating synergistic modification. b) TEM/HRTEM image and FFT. c) Local magnification analysis of the XRD pattern. d) The distribution law of Ti and Nb elements along the radial direction. e) Schematic diagram of synchrotron radiation STXM imaging. f) STXM Mapping diagram. g) XANES spectrum of Ni L-edge. h) XANES spectrum of Ti L-edge
As shown in Figure 1a, the coating-doping modified DC-TNO@SCNCM cathode material was obtained by introducing Ti source and Nb source on the surface of SCNCM, using the sol-gel method for surface coating, and further heat treatment. Transmission electron microscopy (TEM) of Figure 1b shows that the coating thickness is about 15 nm. In the granular phase, the interplanar spacing of the lattice fringes is 0.478 nm, which matches the (003) plane of the SCNCM.
In the coating layer on the particle surface, the lattice fringes with the interplanar spacing of 0.15 nm and 0.50 nm correspond to the (328-) and (104-) crystal planes of the TNO phase, respectively. At the same time, the corresponding Fourier transform also further confirms the above conclusion. In the XRD pattern, the analysis of the (003) and (104) characteristic peaks shows that the diffraction peaks of DC-TNO@SCNCM are shifted to small angles (Fig. 1c), indicating that element doping causes lattice expansion.
To clarify the doping elements, the DC-TNO@SCNCM particles were cut by FIB, as shown in Figure 1d. The Nb element content on the particle surface is relatively high, and the Nb element content decreases sharply to 0 with the deepening of the radial analysis. For the Ti element, its content on the surface is higher, the content of Ti element in the particle is always higher than that of Nb element, and the content of Ti element is always maintained at about 0.25% in the range of 0.1-0.56 μm from the surface.
Figure 1e shows the probing of Ni and Ti elemental distributions and valence states in DC-TNO@SCNCM particles using STXM and X-ray absorption near-edge structure (XANES) spectroscopy. Figure 1g shows that there is a clear Ni L-edge signal in the bulk SCNCM phase. The Mapping map in Fig. 1f shows that Ti is uniformly distributed on the surface of the SCNCM particles. Figure 1h depicts the bulk and surface Ti L-edge XANES in DC-TNO@SCNCM, in which four peaks belong to L3t2g, L3eg, L2t2g and L2eg, corresponding to tetravalent Ti4+, confirming that some Ti4+ is selectively doped into SCNCM.
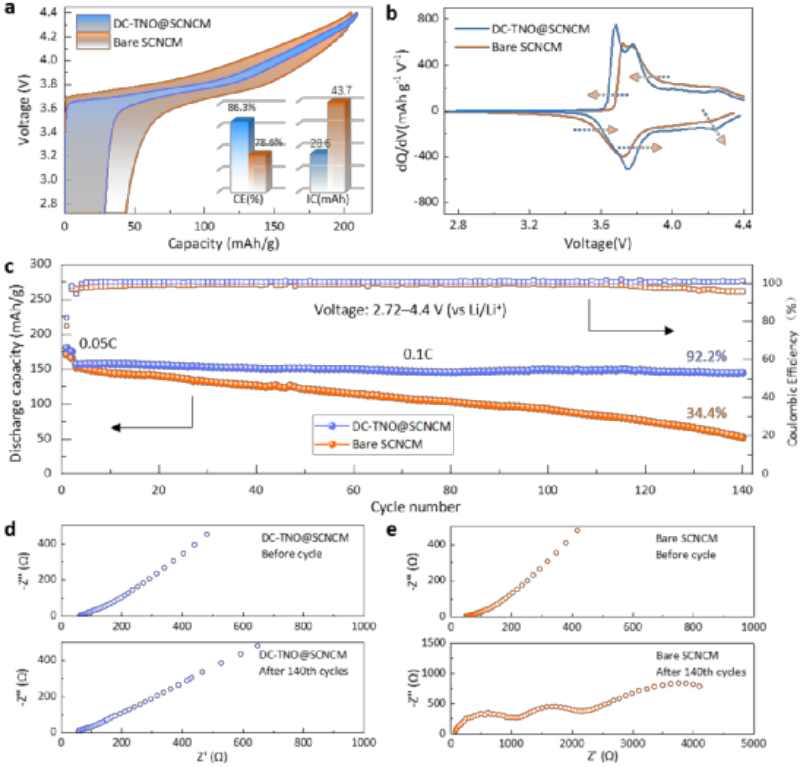
Fig. 2 Comparison of electrochemical performance of DC-TNO@SCNCM and bare SCNCM. a) The first charge-discharge curve. b) The dQ/dV curve corresponding to the first charge and discharge. c) Cycle performance comparison chart. d) Comparison of EIS before and after charging and discharging
The bare SCNCM and the coated-doping modified DC-TNO@SCNCM materials were assembled as cathode active materials to form sulfide all-solid-state battery. Figure 2a shows the first charge-discharge curves of bare SCNCM and DC-TNO@SCNCM. The first discharge specific capacity of bare SCNCM is only 161.1 mAh g-1, relatively speaking, DC-TNO@SCNCM exhibits a high discharge specific capacity of 180.3 mAh g-1.
Meanwhile, the first irreversible capacity of bare SCNCM is 43.7 mAh g-1, while the irreversible capacity of DC-TNO@SCNCM is only 28.6 mAh g-1. The coating-doping modification even increased the first Coulomb efficiency of the ternary material(used to make ternary lithium battery) from 78.6% to 86.3%. In addition, the charge-discharge polarization of DC-TNO@SCNCM is significantly smaller than that of bare SCNCM, which is more intuitively reflected by the dQ/dV curves of the first charge-discharge (Fig. 2b).
As shown in Figure 2c, the clad-doping modified DC-TNO@SCNCM material exhibits more stable cycling performance, with a capacity retention rate as high as 92.2% after 140 cycles. However, with the charge-discharge cycles, the discharge specific capacity of the unmodified bare SCNCM continued to decay, and the capacity retention rate after 140 charge-discharge cycles was only 34.4%.
Electrochemical impedance spectroscopy (EIS, Fig. 2d,e) measurements were performed on the sulfide all-solid-state battery before and after cycling. The results show that the surface-bulk synergistic modification improves the interfacial stability between the cathode and the electrolyte, and improves the high-voltage long-cycle stability of the sulfide all-solid-state battery.
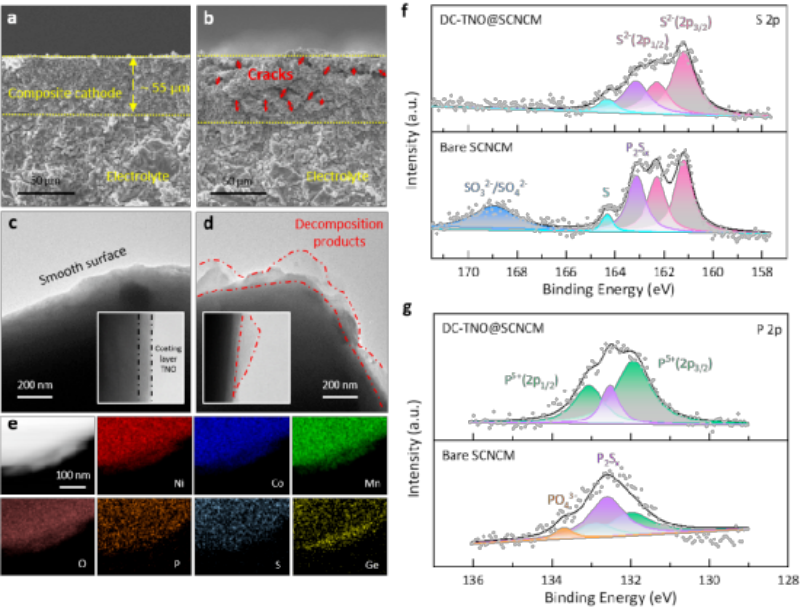
Figure 3 Morphological and chemical information of the electrode after cycling. a) SEM images of the cross-sections of DC-TNO@SCNCM and b) bare SCNCM composite electrodes. c) TEM/HRTEM images of DC-TNO@SCNCM and d) bare SCNCM particles. e) HAADF-STEM image and elemental Mapping of bare SCNCM. XPS images of electrode materials f) S 2p; g) P 2p.
In order to fully understand the effect of the cathode/electrolyte interface state on the electrochemical performance about sulfide all-solid-state battery during the charge-discharge cycle, the cathode after 140 charge-discharge cycles was investigated. The DC-TNO@SCNCM electrode in Figure 3a maintained mechanical integrity, with good contact between electrode parts and no cracks. In contrast, in the bare SCNCM electrode in Fig. 3b, the crack distribution inside the composite cathode can be clearly observed.
Further, the surface of the positive electrode particles after the cycle was observed. As shown in Figure 3c, the surface of the DC-TNO@SCNCM particles is smooth, and the uniform TNO coating layer on the particle surface can be clearly observed. In contrast, after a long cycle of high pressure, the surface of the bare SCNCM particles became uneven, and a large number of by-products were generated on the surface, and the by-products were unevenly distributed on the surface of the bare SCNCM particles.
As shown in Figure 3e, the surface of the bare SCNCM particles is covered with P and S elements. Meanwhile, a large amount of Ge element enrichment was detected in the particle surface layer. P, S and Ge are all representative elements in the electrolyte LGPS. This phenomenon This indicates that the irregular interfacial structure observed on the surface of bare SCNCM particles is generated by the decomposition of the LGPS electrolyte.
XPS results show that the cathode/electrolyte interface formed by direct contact between bare SCNCM and LGPS is unstable, and there are serious side reactions at the interface. The side reaction products mainly include P2Sx, S, SO32-/SO42- and PO43-. Comparative analysis found that the peak intensity of side reaction products in DC-TNO@SCNCM was weaker than that of bare SCNCM, and the interfacial side reactions in reacted DC-TNO@SCNCM were lighter, indicating that the coating-doping modification can effectively Suppressing interfacial side reactions.
In particular, it can effectively avoid the generation of oxygen-containing by-products, including SO32-/SO42- and PO43-. In the composite cathode, the ternary cathode material is the only oxygen source, indicating that the oxygen in the DC-TNO@SCNCM cathode material is more stable, which originates from the bulk Ti4+ doping modification. The comprehensive analysis results show that bulk Ti doping plays a key role in suppressing the interfacial side decomposition reactions.
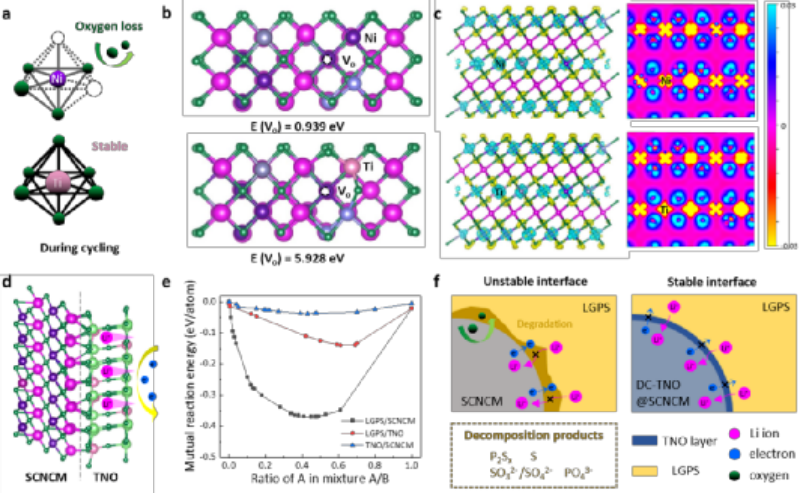
Fig. 4 Mechanism of surface-bulk synergistic modification. a) Schematic diagram of the loss of lattice oxygen during cycling. b) Theoretical calculation of the formation energy of oxygen vacancies in electrode materials. c) Theoretical calculation of the differential charge of the electrode material. d) Schematic diagram of the function of the TNO coating layer. e) Interfacial reaction energy calculation. f) Schematic diagram of the failure mechanism of bare SCNCM interface and the mechanism of DC-TNO@SCNCM coating-doping synergistic modification
The experimental results have demonstrated that the coating-doping modified layered oxide cathode material can stabilize the interface between the layered oxide cathode and the sulfide electrolyte. In order to fundamentally understand the mechanism of coating-doping synergistic modification, first-principles calculations were carried out, hoping to deeply understand the mechanism of coating-doping synergistic modification from the perspective of theoretical calculation.
For bulk doping, the oxygen vacancy formation energy of the material before and after modification was calculated, and DC-TNO@SCNCM showed higher oxygen vacancy formation energy, indicating that the lattice oxygen of the modified material was more stable. Differential charge (CDD) shows that Ti atoms and O atoms exhibit higher charge density, which means that the chemical bond of Ti-O is stronger than that of Ni-O, which once again proves that Ti doping can stabilize O atoms in ternary materials .
For the surface coating, as shown in Fig. 4e, the reaction energies between the various substances were calculated by thermodynamics, and the results showed that the stability of the SCNCM/LGPS interface was poor, showing an obvious decomposition reaction.After TNO insertion, the two interfaces of SCNCM/TNO and TNO/LGPS hardly react, indicating that TNO coating improves the interface stability between SCNCM and LGPS.
According to the experimental and computational results, the modification mechanism of the synergistic modification in DC-TNO@SCNCM is shown in Fig. 4f. The interface of bare SCNCM and LGPS is unstable. During electrochemical cycling at high voltages, interfacial degradation is severe due to the electronic conductivity of SCNCM and the inherently narrow electrochemical window of sulfide SSE. The high oxidation potential leads to the loss of lattice oxygen and the creation of oxygen vacancies, which further oxidize the SSE and form oxygen-containing sulfites and phosphorus-containing species.
The decomposition products severely hinder the ion transport at the interface, thereby affecting the performance of sulfide all-solid-state battery. The significantly enhanced electrochemical performance of DC-TNO@SCNCM can be attributed to two aspects: 1) uniform TNO coating with thermodynamic/electrochemical stability and electronic insulation can avoid SSE decomposition; 2) Ti doping in SCNCM To form strong Ti-O bonds can effectively stabilize the lattice oxygen and prevent the formation of oxygen-containing species at the interface.
3.Conclusion
This study presents for the first time a coating-doping synergistic modification strategy in sulfide all-solid-state battery for high-voltage SCNCM cathode materials. By using the sol-gel method and subsequent heat treatment, a single-crystal NCM cathode material (DC-TNO@SCNCM) with surface TiNb2O7 coating and bulk Ti doping was successfully synthesized. The TNO coating stabilizes the surface, while the Ti4+ doping stabilizes the lattice oxygen of the SCNCM. The TNO coating stabilizes the surface, while the Ti4+ doping stabilizes the lattice oxygen of the SCNCM.

Therefore, the DC-TNO@SCNCM cathode with surface-bulk synergistic modification exhibits a high discharge capacity of 180.3 mAh g-1 with a capacity retention of 92.2% after 140 cycles at a high cut-off voltage of 4.4 V. The excellent electrochemical performance of sulfide all-solid-state battery originates from the ultra-stable interface between cathode and lithium ion battery electrolyte, which shows lower interfacial resistance and fewer interfacial side reaction products. This work provides a novel interface design strategy for the construction of sulfide-based high-voltage sulfide all-solid-state battery, which facilitates the industrialization in sulfide all-solid-state battery.
















