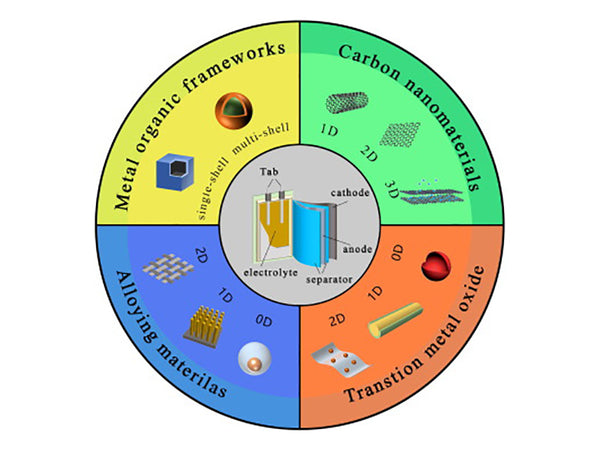
main content:
The negative electrode material of the lithium ion battery is mainly used as the main body of lithium storage, which realizes the insertion and extraction of lithium ions during the charging and discharging process. From the perspective of the development of lithium-ion batteries, the research on anode materials has played a decisive role in the emergence of lithium-ion batteries. It is precisely because of the emergence of carbon materials that the safety problems of metal lithium electrodes have been solved, which directly led to the application of lithium-ion batteries. The negative electrode materials of industrialized lithium-ion batteries are mainly various carbon materials, including graphitized carbon materials and amorphous carbon materials, such as natural graphite, modified graphite, graphitized mesophase carbon beads, and soft carbon (such as coke) And some hard charcoal etc. Other non-carbon anode materials include nitrides, silicon-based materials, tin-based materials, titanium-based materials, alloy materials, and so on. Nano-scale materials are also widely concerned in the research of anode materials due to their unique properties; and the thin film of anode materials is a requirement for high-performance anodes and the development of the microelectronics industry in recent years for chemical power sources, especially lithium secondary batteries.
The development of negative electrode materials for lithium ion secondary batteries has gone through a long process. The earliest researched negative electrode material is metallic lithium. Due to battery safety issues and poor cycle performance, metallic lithium has not been used in lithium secondary batteries. The emergence of lithium alloy solves the potential safety hazards of metal lithium anode to a certain extent, but the lithium alloy has undergone large volume changes during repeated cycles, the electrode material will gradually pulverize, and the battery capacity will rapidly decay, which makes Lithium alloys have not been successfully used as negative electrode materials for lithium secondary batteries. The successful application of carbon materials in lithium secondary batteries promoted the production of lithium-ion batteries, and since then, many kinds of carbon materials have been studied. However, carbon materials have low specific capacity, low first charge and discharge efficiency, and organic solvent co-intercalation. Therefore, while studying carbon materials, people have also begun to develop other high specific capacity non-carbon anode materials, such as tin-based anodes. Materials, silicon-based anode materials, nitrides, titanium-based anode materials, and new alloy materials.
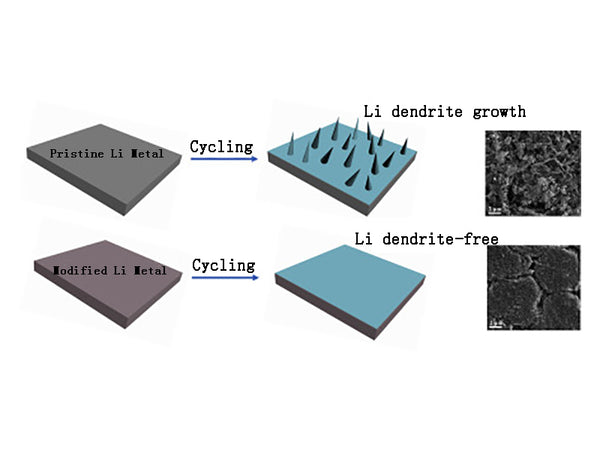
Metal lithium and its alloys
1.Metal lithium and its alloys
The earliest researched lithium secondary battery negative electrode material is metallic lithium, because lithium has the most negative electrode potential (-3.045V) and the highest mass specific capacity (3860mA·h/g). However, when lithium is used as the negative electrode, metal lithium is not uniformly deposited on the electrode surface during the charging process, which causes the lithium to deposit too fast in some parts, resulting in dendritic-like crystals (dendrites). When the dendrites develop to a certain extent, on the one hand, they will break and produce "dead lithium", resulting in irreversible lithium; on the other hand, the dendrites pierce the diaphragm, causing internal short circuits and battery explosions. In addition, lithium has great reactivity, which may react with the electrolyte, and may also consume active lithium and cause safety problems. It is precisely because of the many problems that may be caused by the lithium dendrites and the reaction between lithium and the electrolyte, the secondary lithium batteries using lithium as the negative electrode have not been commercialized. At present, work is mainly carried out in three areas: ①Finding alternative anode materials for metal lithium; ②Using polymer or molten salt electrolyte to avoid the reaction of metal lithium and organic solvents; ③Finding a suitable electrolyte formula to make metal lithium in the deposition and dissolution process Maintain a smooth and uniform surface.
Historically, systematic research on lithium alloys began with high-temperature molten salt systems. The research systems include Li-Al, Li-Si, Li-Mg, Li-Sn, Li-Bi, and Li-Sb. The systematic study of chemical alloying reactions started from Dey's work. Later studies have shown that lithium can undergo alloying reactions with many metals in the electrochemical process at room temperature. Huggins has done a systematic study on the behavior of various binary and ternary lithium alloys as negative electrodes in organic solvent systems, especially the thermodynamic and kinetic behaviors of lithium tin systems, lithium antimony systems, and lithium lead systems.
Compared with metal lithium, the lithium alloy negative electrode avoids the growth of dendrites, thereby improving safety. However, as the alloy material undergoes a large volume change during repeated cycles, the electrode material will gradually become powdered and the battery capacity will rapidly decay.
In order to solve the powdering problem of alloy materials, different researchers have proposed different solutions. Huggins proposed to uniformly disperse the active LixSi alloy in the inactive (the so-called inactive refers to not participating in the reaction at a certain potential) LixSn or LixCd to form a mixed conductor all-solid composite system. It has been proposed to disperse lithium alloys in conductive polymers to form composite materials; to embed small-particle alloys into a stable network support. These measures inhibited the powdering of alloy materials to a certain extent, but still did not meet the requirements of practicality.
With the breakthrough of the negative electrode concept, the negative electrode material no longer needs to contain lithium, which allows more choices in the preparation of alloy materials.
Lithium-free intermetallic compounds are used in lithium-ion battery anodes for research. There are two types of intermetallic compounds. One is the intermetallic compound between two lithium-intercalable alloys, such as SnSb, SnAg, AgSi, GaSb, AlSb, and InSb. This type of intermetallic compound, because different metals are in different The potential and lithium undergo an alloying reaction. When one metal and lithium undergo an alloying reaction, the other metal is inert, which is equivalent to an active alloy dispersed in a network of inactive alloys. Compared with a single metal, the cycle performance of the material is greatly improved. Another type of intermetallic compound is an alloy of active and inactive metals that can insert lithium, such as Sb2Ti, Sb2V, Sn2Co, Sn2Mn, Al2Cu, Ge2Fe, CuSn, Cu2Sb, Cr2Sb. Only one metal of this type of alloy is active, and the other one acts as a conductive inert network. Compared with the former two active metals, the intermetallic compound cyclability is improved, but this is at the expense of specific capacity. of.
In addition, the introduction of multi-phase alloys has also improved the cycle of materials, such as Sn/SnSbx, Sn/SnAgx, SnFe/SnFeC, SnMnC.
Intermetallic compounds have not completely solved the problem of material powdering, and people have begun to pay attention to small-sized materials. Besenhard found that the fragmentation of sub-micron or nano materials in the circulation process becomes smaller, and the circulation of the material becomes better as the particles decrease. This is due to the small absolute volume change of nanomaterials during charging and discharging, and the pulverization of materials can be well suppressed. However, due to the large surface area and surface energy of nanomaterials, there are serious electrochemical agglomeration problems during the electrochemical cycle. Someone has done research on the capacity loss and capacity decay of nano-tin-bronze alloy in lithium-ion batteries. It is believed that the first capacity loss of nano-alloy and the capacity decay during cycling are mainly caused by 5 reasons: surface oxide, electrolyte Decomposition, lithium capture by the host material, presence of impurity phases, agglomeration of active particles during electrochemical cycling.
Another noteworthy research result in alloys is Fuji Film's use of tin-based composite oxide (TCO) as the negative electrode of lithium-ion batteries. The glassy tin-based composite oxide negative electrode has good cyclability.
Carbon materials
2.Carbon materials
Research on lithium alloys has not directly led to the production of lithium-ion batteries, and non-lithium alloys have been studied before and after the emergence of lithium-ion batteries. What really promotes the emergence of lithium-ion batteries is the application of carbon materials in lithium-ion batteries.
The study of carbon materials as lithium-ion batteries began in the 1980s, but the lithium insertion behavior of carbon materials has been studied before then. As early as the mid-1950s, Herold synthesized Li-graphite intercalated compound (GIC, graphite intercalated compound). In 1976, Besenhard discovered that lithium can be electrochemically intercalated into graphite from non-aqueous solutions. However, due to the expansion of the graphite structure and the disintegration of the macrostructure during the charging and discharging process, this problem has not been solved. In the early 1980s, someone reported the study of combining lithium with carbon immersed in molten lithium and discovered that LiC6 can be used as the negative electrode of the battery, which opened the prelude to the study of carbon as the negative electrode of lithium-ion batteries. In 1985, Sony Corporation of Japan proposed to use disordered non-graphitized carbon as the negative electrode of the battery, thus inventing the lithium-ion battery. After that, Sony successfully launched a lithium-ion battery with LiCoO2 as the positive electrode and polyfarfury alcohol (PFA) pyrolysis carbon (hard carbon) as the negative electrode, thus commercializing the lithium-ion battery. Table 1 shows the development process of different carbon materials.
Table 1 Historical background of different carbon materials
| years | History background |
| 1976 | Discovery of Electrochemical Intercalation Behavior of Alkali Metal Ions in Organic Donor Solvents |
| 1981 | The emergence of molten salt batteries with LiC6 as the negative electrode, NbSe3 as the positive electrode and DOL as the solvent |
| 1983 | A polymer battery with lithiated graphite as the negative electrode and LiClO4/PC as the electrolyte |
| 1985 | Introduction of disordered non-graphitized carbon as anode material |
| 1990 | Commercial battery-Li/MnO2 electric pair with hard carbon as negative electrode |
| 1990 | Coke is used as the negative electrode, LiMnO2 is used as the positive electrode, and the electrolyte is LiAsF6 /(EC+PC) |
| 1993 | Introduction of graphitized MCMB and non-graphitized VGCF as anode materials |
Note: DOL-dioxalane, dioxolane; PC-propylene carbonate, propylene carbonate; EC-ethylene carbonate, ethylene carbonate; MCMB-mesocarbon microbeads, mesocarbon microbeads; VGCF-vapour grown carbon fibre, vapor growth carbon fiber.
The lithium intercalation behavior of graphite-like carbon materials has been thoroughly studied and has been recognized by everyone. The carbon atoms in graphite are sp2 hybridized and form a lamellar structure. The layers are combined by van der Waals forces, and the atoms in the layers are covalently bonded. D.Guerard et al. chemically inserted lithium between the layers of the graphite sheet structure to form a series of intercalation compounds, such as LiC24, LiC18, LiCl2, and LiC6. J.R.Dahn also proved that lithium-graphite intercalation compounds are formed by electrochemical methods, and a series of intercalation compounds are formed during the lithium intercalation process. Due to the weak van der Waals forces between the graphite sheets, during the electrochemical intercalation reaction, the partially solvated lithium ions will also bring solvent molecules into the intercalation process, resulting in the co-intercalation of the solvent, which will gradually peel off the graphite sheet structure. . This is particularly obvious in the electrolyte system with PC as the solvent. This is also the reason why the first-generation lithium-ion battery filed by Sony did not use graphite but instead used coke with an amorphous structure. Later, due to the emergence of mesophase carbon microbeads (MCMB) and the use of ethylene carbonate (EC)-based electrolytes, graphite-based carbon materials became the negative electrode materials for commercial lithium-ion batteries.
In addition to graphite, another major category of carbon materials is amorphous carbon materials. The so-called amorphous refers to the lack of a complete lattice structure in the material. Similar to the arrangement of atoms in a glassy structure, there is only a short order and no long order. Amorphous carbon materials are between graphite and diamond, and there are sp2 and sp3 hybrids of carbon atoms.

oxide anode material
3.Oxide anode material
The oxide mentioned here does not include the oxides of metals that can form alloys with metallic lithium, such as tin and lead.
The oxide negative electrode material must start with the high-temperature battery in the 1980s. The discharge platform of α-Fe2O3 and Fe3O4 in high temperature batteries (420℃) is 0.8~1.1V, and the capacity can reach 700mA·h/g. The gradual deterioration of battery performance may be caused by the gradual diffusion of lithium oxide into the electrolyte. . X-ray diffraction results show that α-Fe2O3 irreversibly transforms from corundum structure to spinel Fe3O4 structure during the discharge process, and finally forms γ-Fe2O3. During the oxidation process, γ-Fe2O3 is finally formed through the Fe3O4 mesophase. Then in 1985 B.Scrosati et al. reported the electrochemical behavior of iron oxide in lithium organic solvent rechargeable batteries. At the same time, P.Novak reported the electrochemical behavior of copper oxide in lithium batteries. In 1993, Idota discovered that materials based on vanadium oxides can insert 7 lithium atoms per molecule at a lower potential, with a capacity of 800-900 mA·h/g, and good cyclability. This renewed interest in the application of oxygen-containing materials in lithium-ion batteries. JMTarascon et al. studied the reversible reaction mechanism of vanadate and believed that the material formed a composite material of nano-metal particles and lithium oxide during the first discharge process. Under the catalysis of the nano-metal particles, the lithium-oxygen bond in the lithium oxide was reversibly broken. And formation is the source of the reversible capacity of the material. Transmission electron microscopy proved that the mechanism of copper oxide being reduced by lithium includes the formation of a solid solution CuII1-xCuIxO1-x/2 (0<x<0.4), then a phase transformation to form cuprous oxide, and then the formation of copper dispersed in the lithium oxide grid It is believed that the oxide lithium storage process is mainly due to the reversible formation and decomposition of lithium-oxygen bonds due to the high activity of nano-copper or other 3d metal particles. For the reversible fracture and formation mechanism of lithium-oxygen bonds proposed by the Tarascon group, JRDahn In-situ X-ray diffraction and Mssbauer spectroscopy studies have shown that the oxide undergoes an electrochemical displacement reaction that rapidly decomposes to form lithium oxide and metal during the discharge process, and the reaction product is a nano-scale metal. During the charging process, the metal is first oxidized, and then the oxidized metal replaces the lithium in the lithium oxide to form a metal oxide and lithium. For example, in CoO, this reaction does not change the oxygen lattice in lithium oxide during charging, which is a bit like an ion exchange reaction. This phenomenon also exists in iron oxide. During discharge, it is as if lithium ions replace metal atoms in the oxide. In subsequent cycles, this exchange reaction proceeds reversibly. The current lithium insertion mechanism of oxides is still controversial, but this does not prevent us from using oxides to prepare new electrode materials. J.R.Dahn et al. studied the lithium insertion behavior of composite materials obtained using lithium oxide or lithium sulfide and metal nanoparticles. The material shows electrochemical activity and has a capacity of 600mA·h/g. When the potential limit is appropriate, the cycle capacity of the material does not decay.
Other oxide anode materials also include MO2, MnO2, TiO2, VO2, CrO2, NbO2, MoO2, WO2, RuO2, OsO2, IrO2, α-MoO3 and other materials with a rutile structure.
negative electrode materials
4.Other negative electrode materials
Transition metal nitrides are another type of negative electrode material that has attracted widespread attention. TakeshiA.sai et al. reported the preparation and ionic conductivity properties of CuxLi1-xN in 1984, and the lithium copper nitrogen obtained by replacing part of the cations in Li3N. Due to the partial covalent bond between copper and nitrogen, the activation energy is reduced to 0.13 eV. In addition, due to substitution, the lithium vacancy is reduced, and the lithium ion conductivity is reduced. The O.Yamamoto team conducted in-depth research on the electrochemical lithium insertion process of Li7FeN2, Li7MnN4, Li2.6M0.4N (M=Co, Ni, Cu) materials, and found that these materials have a capacity of up to 900mA·h/g, and It has good circulation. Other groups have also done a lot of work on nitrides. Since the lithium-containing negative electrode is not applicable in the current lithium-ion battery system, other factors, such as preparation cost and sensitivity to air, are still far from practical application, but it provides another choice of electrode materials. It is also a good attempt to compensate for the first irreversible capacity loss in combination with other electrode materials.
Others, such as borate, fluoride, sulfide, etc., have also been reported for the study of anode materials for lithium-ion batteries. AlaZak et al. studied the situation of alkali metal intercalation of metal sulfide (WS2, MoS2) nanoparticles with a fullerene structure. The sealing layer on the surface is the main limiting factor for lithium insertion.

Other negative electrode materials
5. Composite anode material
At present, the negative materials used in commercial lithium-ion batteries are all carbon materials, including graphitized carbon materials such as graphitized mesophase carbon microbeads (MCMB) and some pyrolytic hard carbons. At present, the actual specific capacity of these carbon materials generally does not exceed 400mA·h/g. Although it is higher than the specific capacity of most of the current cathode materials (usually 120~180mA·h/g), due to the compactness of carbon materials The density is low, and the general negative electrode current collector uses heavy copper foil and the positive electrode uses lighter aluminum foil, so the actual volume of the positive electrode material is higher than the negative electrode; therefore, the specific energy of the battery should be further improved, and the negative electrode material’s Lithium insertion performance is the key to research and development. Moreover, with the increasing popularity of electronic products, the demand for high specific energy batteries is getting higher and higher. At present, no single material can fully meet the relevant needs. Although carbon materials have good cycling performance, their specific capacity is low; other electrochemical properties of carbon materials with high specific capacity are impaired. The alloy material has a high specific energy, but due to the large volume expansion during the lithium insertion process, the cycle performance of the material is far from meeting the requirements. Tin-based composite oxide has good cycle characteristics, but the first irreversible capacity loss has not been solved. From this point of view, it is a reasonable choice to combine the advantages of various materials and combine various materials purposefully to avoid their own shortcomings. The formation of composite anode materials is a reasonable choice. The current research on composite materials has achieved certain results.
Regarding the first irreversible capacity loss of materials, it was proposed to use lithium-containing transition metal nitrides to compensate, and to use lithium and tin oxide to react to solve the first irreversible capacity loss of tin oxide materials.
Aiming at the problem of poor cycleability of alloy materials, some people have proposed the idea of dispersing an active material in another inactive material to form a composite material. Such efforts include the use of inert grids formed by excess copper proposed by Thackeray et al. to improve the electrochemical cycling of copper-tin alloys. Hisashi Tamai et al. used organotin to prepare a composite material with nano-scale tin dispersed in a carbon grid to improve the material's recyclability. For example, graphite-tin composites were prepared by ball milling; composite materials composed of conductive polymers/metal alloys were studied; carbon was coated on the surface of silicon particles by the CVD method, and it was found that the electrochemical cycling of silicon after surface coating was great Improved, the silicon particles did not break after repeated cycles; conductive polymer and lithium alloy composite electrodes were prepared. These all significantly improve and enhance the electrochemical cycling of alloy materials to a certain extent.
















