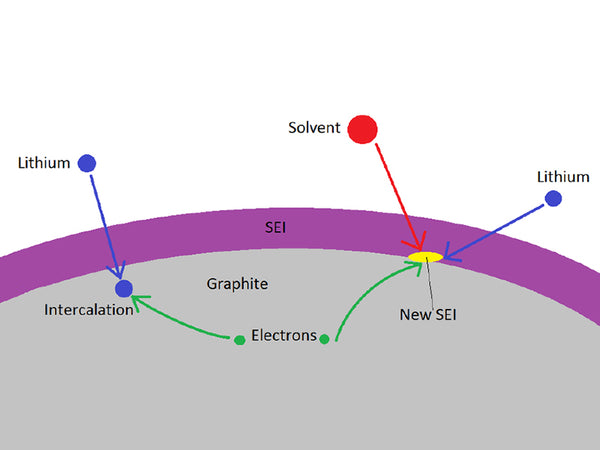
During the first charge and discharge of lithium-ion batteries, that is, before lithium ions begin to intercalate into the graphite electrode (>0.3V), the organic electrolyte will be reduced and decomposed on the surface of the carbon negative electrode to form a passivation layer that is electrically insulating and ionically conductive. This passivation layer is called a solid electrolyte interface (solid electrolyte interface, SEI) film. The formation of SEI film on the surface of carbon material before electrochemical lithium insertion has been confirmed. In addition, some other negative electrode materials, such as tin oxide and alloy surface can also form SEI film; moreover, the surface of positive electrode material can also be anodicized The SEI film is formed.
The formation of SEI film consumes limited Li+ on the one hand and reduces the reversible capacity of the battery; on the other hand, it also increases the interface resistance of the electrode and electrolyte, which increases the polarization of the electrode and affects the high-current discharge performance of the battery. . However, the excellent SEI film is insoluble in organic solvents, allowing lithium ions to freely enter and exit the carbon negative electrode while the solvent molecules cannot pass through. It can effectively prevent the further reaction between the organic electrolyte and the carbon negative electrode and the co-intercalation of solvent molecules to damage the carbon negative electrode. The cycle efficiency and reversible capacity of the battery.
SEI film formation process
Since the insertion process of lithium ions must go through the SEI film covering the carbon anode, the characteristics of the SEI film determine the kinetics of lithium insertion/desorption and the stability of the carbon anode/electrolyte interface, which also determines the performance of the entire battery, such as Cycle life, self-discharge, rated rate, and low temperature performance of the battery. To optimize the properties of the SEI film at the electrode interface, it can be achieved by improving the properties of the electrode interface and optimizing the electrolyte composition.
(1) Composition and structure of SEI film
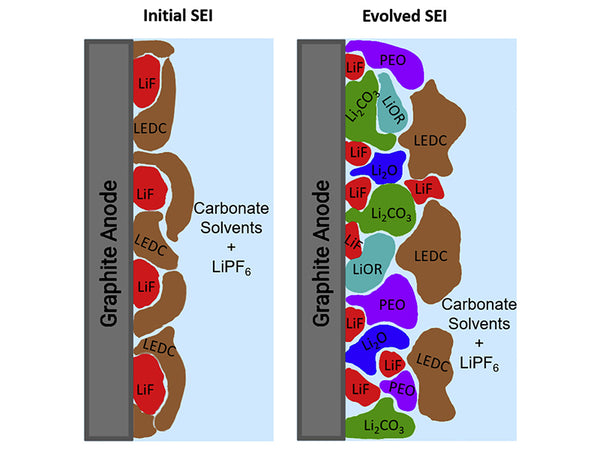
The interface reduction products of the graphite electrode in the first discharge process of the PC/EC-based electrolyte are C2H4, propylene, and Li2CO3; during the reduction process of the PC and EC-based electrolytes at the electrolyte electrode interface, the reaction is terminated by a single-electron radical to form lithium alkyl carbonate. (ROCO2Li); In addition, polypropylene oxide P(PO)x is formed in the SEI film formed by the graphite anode in the PC-based electrolyte; some people are studying the petroleum coke in the LiCF3SO3-PC/EC/DMC electrolyte. The specific composition of the SEI film at the interface is Li2CO3, ROCO2Li, ROLi, and the other components are unknown, and may be one or more of L2S, LiF or Li2SO3.
The composition of the SEI membrane is very complex, mainly composed of electrolyte components, including organic solvents, potassium salt electrolytes, additives or possible impurities, such as H2O, HF and other reduction products on the electrode surface. In addition, the electrode material The composition also affects the composition of the SEI film. Therefore, the composition of the SEI film is also different if the solvent is different, or the lithium salt electrolyte is different.
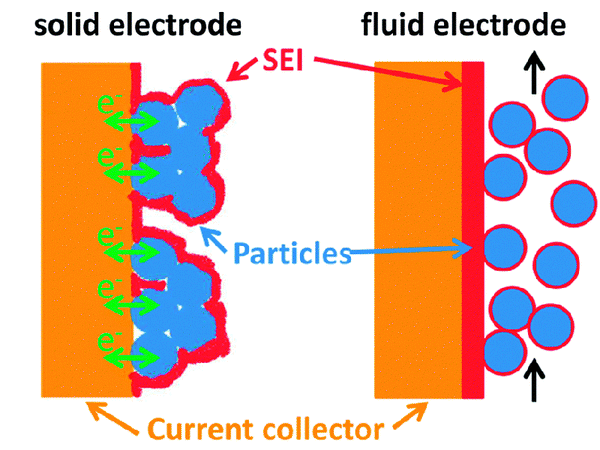
SEI membrane in solid and liquid electrolyte
In terms of solvent, in the alkyl carbonate electrolyte, the SEI film is mainly composed of ROCO2Li. For the chain solvent, it is alkyl monolithium carbonate, and the cyclic solvent is alkyl dilithium carbonate; for EC- For DMC-based electrolytes, some lithium alkoxides may also be present in CH3OLi. In the mixed solvent system of alkyl carbonate ether, the main component of the SEI film is lithium alkyl carbonate. A small amount of water in the electrolyte will also react with lithium alkyl carbonate to form more stable Li2CO3.
In terms of electrolyte, in a fluorine-containing electrolyte, the HF generated by the decomposition of the lithium salt will react with the surface components to form LiF, or the lithium salt will be directly electrochemically reduced to form LiF. Taking LiAsF6 as an example, the possible electrochemical reduction is as follows :
LiAsF6+2Li++2e→2LiF+AsF3
AsF3+Li++e→LixAsFy+LiF
In the LiClO4 electrolyte, there are corresponding reduction products Li2O and LiCl, etc.
LiClO4+8e+Li+→4Li2O+LiCl
Or LiClO4+4e+4Li+→2Li2O+LiClO3
Or LiClO4+2e+2Li+→Li2O+LiClO3
LiPF6 is correspondingly reduced to LiF and LixPFy; N(SO2CF3)2- is reduced to LiF, lithium nitride and lithium sulfide, such as Li2S, Li2S2O4, Li2SO3.
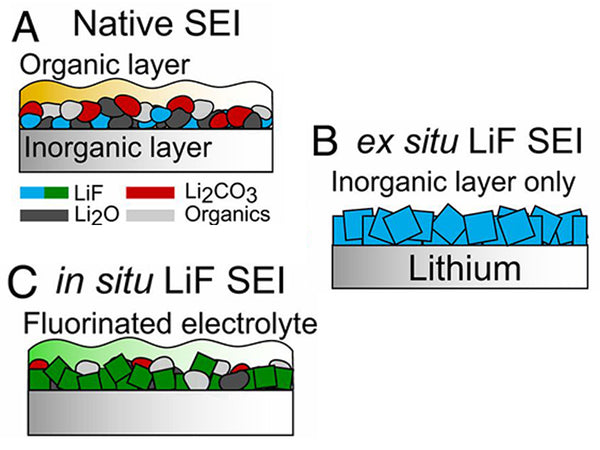
Lithium fluoride in the decomposition interface
Therefore, the main components of the SEI film include lithium alkyl carbonate, lithium alkoxide, lithium halide, Li2CO3, Li2O, lithium sulfide, and the like.
The passivation film structure model established by Peled believes that the passivation film contains 1 to 2 molecular layers. The first layer is thin and dense, which is an important reason to prevent further reduction of electrolyte components. If the second layer exists, it will cover On the first layer, there is often a porous structure. Later, Thevenin revised this model. The main points are as follows: ①The passivation film formed by the electrode in the PC-based electrolyte is polypropylene oxide P(PO)x formed by the polymerization reaction initiated during the reduction of PC and some simple Lithium salt, such as Li2CO3, etc.; ②The solid compound is dispersed in the polymer network to form a solid network structure; ③The passivation film is a thin bimolecular layer, one layer is closely connected with the electrode interface, and the structure is dense; the other layer may be The dense molecular membrane layer may also be a porous molecular membrane layer. The shortcomings of the above-mentioned SEI membrane structure model are that one is that it cannot explain the high interface impedance of the SEI membrane; the other is that the remarkable thermodynamic and kinetic stability of the interface cannot be consistent with the high chemical activity of the membrane components at the electrode interface; The hypothesis of molecular layer or bimolecular layer does not match the actual thickness (5~10nm) of general SEI film.
It was proposed that the SEI film contains five continuous molecular layers in structure, and there is an interface between two adjacent layers, and each interface is a lithium-conducting pathway. In this way, according to the model, there are 5 continuous Li+ transfer channels, and each layer has a corresponding Li+ capacity and resistance. It is difficult to determine the structural and conductive properties of each layer, such as dielectric constant, ionic conductivity, and electrical conductivity. The activation energy is distinguished from the other layers; the important reason for the high impedance of the interface is the boundary resistance Rgb of the crystallites, because Rgb is related to the difficulty of ion migration from one particle to another particle perpendicular to the direction of the ion current. Later, it was confirmed by XRS method that the SEI film is a multilayer molecular interface film, the components closely connected to the electrode interface are relatively stable anions O2-, S2- or F-, and the components closely connected to the electrolyte are partially reduced products. Such as polypropylene components. Peled believes that it is more appropriate to regard the SEI film as a polymerized state of multiple particles. Each particle has corresponding ionic resistance and boundary lithium conductivity. The film thickness is 5-50nm. This multilayer SEI film can be better simulated The interface AC impedance characteristics of the SEI film.
(2) The formation mechanism of SEI film and the mechanism of conducting lithium
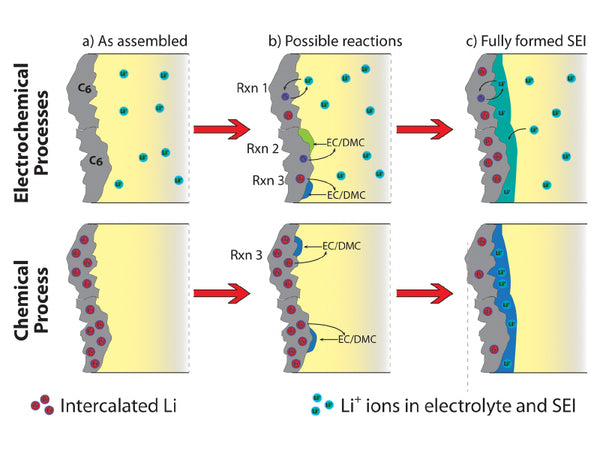
There are two physical models for the formation mechanism of SEI films on graphite electrodes. Besenhard et al. believe that: the solvent can be co-embedded in graphite to form a ternary GIC, and its decomposition products determine the impact of the above reaction on the performance of graphite electrodes; the reduction products of EC can form a stable SEI film, even in the graphite structure; The decomposition products impose an interlayer stress in the graphite electrode structure, causing the destruction of the graphite electrode structure (referred to as delamination). Another model was developed by Aurbach et al. after Peled, based on the spectral analysis of the decomposition products of electrolyte components. This model believes that the formation of the initial SEI film controls the characteristics of further reactions, and the delamination of the graphite electrode at the macro level is caused by the poor passivation performance of the initially formed SEI film and gas decomposition products. The biggest difference between the two mechanisms is that the first step of SEI film formation starts with the formation of a ternary graphite intercalation compound or the electrochemical reduction of the electrolyte on the surface of the graphite electrode.
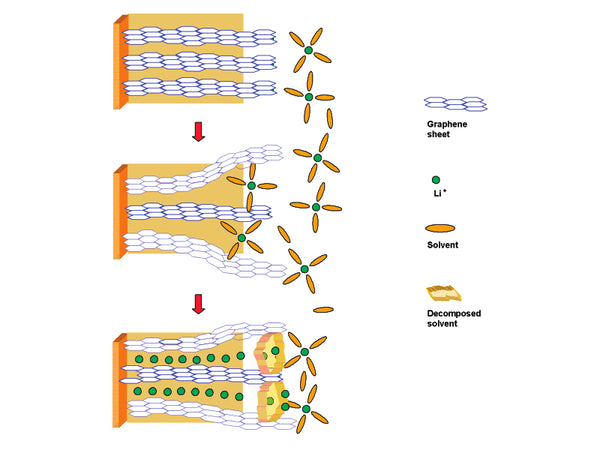
Schematic diagram of SEI formation mechanism
Combining the above two models, Chung et al. proposed another SEI film formation mechanism that includes solvent co-intercalation: the formation process of the SEI film on the surface of the graphite electrode starts with the most easily reducible electrolyte composition until the charge is transferred to the electrolyte. The speed of the components becomes very slow. If the reduction products of the electrolyte components still cannot establish an SEI film with excellent passivation performance on the electrode surface, when the potential is as low as the ternary GIC and the thermodynamic energy is stable, the SEI film The formation is carried out by the reduction of the ternary GIC compound. When the interlayer stress generated by the ternary GIC compound exceeds the mutual attraction force between the graphite layers, the graphite will delaminate. When the graphite electrode has very superior mechanical integrity or the ternary GIC compound has a small stress, the graphite electrode will not Delamination occurs, so that the SEI film can continue to form through a series of electrolyte reduction reactions. The structure of the electrolyte solvent and the properties of the graphite body are the two main factors that affect the delamination of the graphite electrode.
The reduction reaction of solvent molecules at the electrode interface can be divided into chemical reduction and electrochemical reduction. Electrochemical reduction refers to the process in which the solvent molecules combined with Li+ obtain electrons at the electrode interface and are reduced. According to the number of electrons obtained by each solvent molecule in the electrochemical reduction process, it is divided into single-electron reduction mechanism and two-electron reduction. mechanism. Chemical reduction refers to the reduction process that Li+ obtains electrons on the electrode and becomes Li/C intercalation compound, based on the reduction effect of the electrode itself, and the reduction process with solvent molecules. Generally speaking, chemical reduction is very weak at the carbon anode/electrolyte interface. Now take PC as an example to introduce the solvent reduction reaction.
Electrochemical reduction reaction:
2PC+2Li++2e→CH3CH(OCH2Li)CH2OCO2Li+CH3CHCH2↑ (single electron mechanism)
PC+2Li++e→LiCO3+CH3CHCH2↑ (dual electron mechanism)
Chemical reduction reaction:
2PC+Li+Cn-→Li2CO3+CH3CHCH2↑+Cn
The electrochemical reduction mechanism of the solvent is briefly described below.
In lithium-ion batteries, the initial step of electrochemical reduction is the transfer of electrons from the cathode-polarized electrode to the solvated lithium ions, so that the free radicals of the solvent molecules produced can interact with the surrounding solvent molecules that complex with the lithium ions. A charge exchange balance is established between, and the subsequent solvolysis starts from this charge exchange balance. The charge transfer from the electrode to the solvent molecule coordinated with the lithium ion requires an empty molecular orbital for charge transfer. If the energy of this empty orbital is higher, or its lowest unoccupied molecular orbital (LUMO) energy When it is higher, the transfer of charge can only be carried out at a lower potential. Since graphite electrodes are usually lithiated under constant current conditions, the formation process of the SEI film on the surface is a highly selective process. In other words, the most reactive substance is first reduced at a higher potential and its reduction product is deposited on the surface of the carbon negative electrode to form an SEI film, which inhibits the reduction of other solvents with lower activity, which has been reduced by different potentials. The following research confirms the formation of the graphite electrode surface film.
The potential (VSEI) formed by the SEI film is related to the temperature, the concentration of the reduced substance, the solvent, the lithium salt, the current density and the carbon boundary and the catalytic activity.
There are two mechanisms for the transmission of lithium ions in the SEI film, one is the ion exchange mechanism, and the other is the ion migration mechanism. It is still unclear which mechanism is more convincing. The ion exchange theory believes that Li+ reaches the SEI membrane and exchanges cations with Li+ in the components of the SEI membrane, thereby realizing the transfer of Li+; while the ion migration mechanism is that Li+ in the liquid phase migrates through the SEI membrane to the electrode body.
If the lithium-conducting mechanism of the SEI film is an ion exchange mechanism, the shorter the relaxation time of the cation exchange reaction of the lithium salt in the electrolyte, the better the lithium-conducting property. This mechanism corresponds to a cation migration number of 1, and an electron migration number of 0, and the concentration distribution of the charged carrier has nothing to do with the film thickness, that is, ∂n/∂x=0; in the entire electrode reaction process, the Li+ transmembrane transfer process will determine the rate of the entire electrode reaction. In this sense, the order of ion conductivity of common SEI membrane components is: Li2SO3>Li2CO3>ROCO2Li>ROLi. Experiments have shown that adding SO2 and CO2 to the electrolyte can greatly improve the charge and discharge performance of the electrode, and the effect of adding SO2 is better than that of CO2, and the conductivity of Li2CO3 is greater than that of ROCO2Li. The presence of trace amounts of water (5×10-4) in the DMC-based electrolyte not only does not have any destructive effects on the performance of the graphite electrode, but also shows a large improvement. These facts can be used as a basis for supporting the ion exchange mechanism.
Studies have shown that the diffusion coefficient D of Li+ in the carbon anode is about 10-5cm²/s, which is approximately equal to the diffusion coefficient of Li+ in the liquid phase, which is much larger than the diffusion coefficient of positive ions in many crystals (D≈10-12~10-9cm²/s); this result contradicts the high interface resistance of the ion exchange transfer mechanism. In this sense, the mechanism of Li+ migration through the micropores of the SEI membrane seems more realistic. If the lithium-conducting mechanism of the SEI film is the ion migration mechanism. Then, the conductivity of the membrane comes from the conductivity of the remaining electrolyte in the micropores of the membrane structure, which is inversely proportional to the electrode surface coverage θ, the cation migration number is less than 1, and the concentration distribution of the charged carrier in the membrane structure is related to the thickness of the membrane (∂n /∂x≠0), the volume and structural arrangement of the membrane components seem to have a greater impact on the conductivity of Li+.
Based on this, the selection principle of SEI film with excellent performance is as follows.
①The formed electrochemical potential is higher than the intercalation potential of solvated Li+ to prevent the co-intercalation of solvent molecules from damaging the electrode;
②The number of electron migration is 0, which has excellent electronic insulation to avoid the continuous growth of the film leading to high internal resistance of the battery;
③The migration number of Li+ is 1 to eliminate the concentration polarization near the electrode and facilitate the rapid deintercalation of Li+;
④The membrane thickness is small, the relaxation time of the cation exchange reaction of the membrane components is short, and the lithium conductivity is good, which can reduce the overpotential of the battery charging and discharging process;
⑤It has good thermal and chemical stability. The upper and lower temperature limits for the stable existence of the SEI film are wide. The SEI film does not react with the electrolyte and the electrode active material or is insoluble in the electrolyte;
⑥Excellent chemical bonding structure with electrode interface, good dynamic stability;
⑦ Uniform morphology and chemical composition are conducive to the uniform distribution of current;
⑧ Sufficient adhesion, mechanical strength and toughness.
















