main content:
Polymer electrolytes are usually composed of lithium salt (LiX) and polymers with high molecular weight such as (PEO), but PEO is easy to crystallize below 60°C, and the rapid transport of ions must be carried out in the amorphous region. Therefore, PEO-LiX The conductivity of the electrolyte needs to be between 60~80℃ to reach a practical conductivity value (10-4S/cm). The most common method is to add a plasticizer to the polymer electrolyte to reduce the use temperature, but this will reduce the mechanical properties of the polymer electrolyte and increase its reactivity with the lithium metal negative electrode.
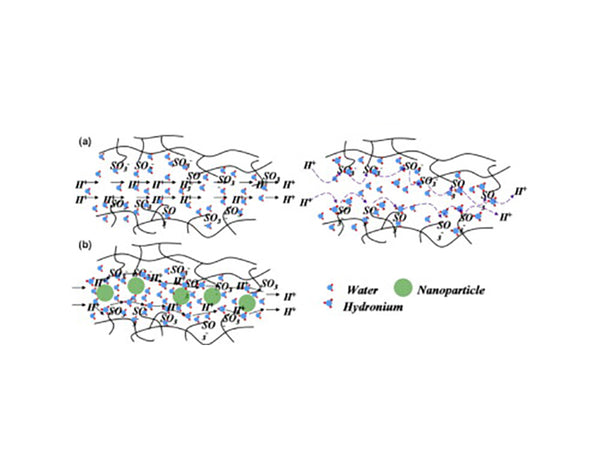
Figure 1 Polymer-nano composite electrolyte membrane
1. Research progress of nano-ceramic composite polymer electrolytes

Ceramic powder with nanometer size is used as a solid plasticizer for PEG-based electrolyte, which is called nanocomposite polymer electrolyte. For example, after adding TiO2 and Al2O3 powder with a particle size of 5.8~13nm, the conductivity of the PEO-LiClO4 mixture can reach 10-4S/cm at 50℃, and 10-5S/cm at 30℃, because the nano-ceramic powder is driven by power In terms of science, the polymer electrolyte was prevented from transforming from the amorphous phase to the crystalline phase during annealing above 60℃. The effect of the addition of nano-ceramic powder on the PEO-LiClO4 system was studied by 7Li NMR, and it was found that the electrical conductivity and ion mobility of the system are both However, TiO2 has the greatest impact on the polymer electrolyte; the increase in the number of cation migration is directly related to the increase in the Lit diffusion rate; the increase in conductivity is not due to the increase in the movement of polymer segments, it is probably due to the presence of nanoparticles It is caused by weakening the interaction between the cation and the ether oxygen. For example, if LiAlO2 ceramic powder is added to the PEO-LiClO4 system, the electrical conductivity of the system is 3.5×10-5S/cm at 60°C. XRD and 7Li NMR tests show that the addition of LiAlO2 improves the mobility of ions in the composite system.
Adding γ-Al2O3, SiO2, LiAlO2 and other ultra-fine inorganic powders to the polymer electrolyte will destroy the crystal structure of the polymer, increase the content of the amorphous region, or delay the recrystallization rate, so that the ionic conductivity of the polymer is better. Big improvement. The introduction of nano-scale inorganic fillers such as Al2O3, TiO2 and SiO2 can effectively strengthen the toughness of the electrolyte and improve the Its electrochemical properties. In some cases, with the increase of nano-fillers, the T of the nano-scale polymer electrolyte increases instead. For example, in some polyacrylonitrile (PAN)-lithium perchlorate (LiClO4) electrolytes, the increase in ionic conductivity is not due to the corresponding increase in the movement of the polymer, which is caused by the introduction of nanoparticles. Caused by the weakening of the interaction. For many polymer electrolyte systems based on PEO, it shows that there is no direct and obvious interaction between the filler particles and the polymer. In polycrystalline and polymer particle solid electrolytes, the polymer-ceramic particle interface does not contribute to the ionic conductivity, but the particle interface impedance related to the interface between the ions passing through the particle interface and the current direction perpendicular to the direction of the current cannot be ignored.
Adding 10% nano Al2O3 to PEO-LiBF4, the conductivity can reach 10-4S/cm at room temperature. The conductivity of the composite of the same amount of micron inorganic particles and polymer is only 10-5S/cm. Reducing the particle size can increase the interface layer between the inorganic ions and the polymer. Therefore, the increase in ion conductivity is closely related to the formation of the interface layer. Nano-ceramic additives can affect the physical and electrochemical properties of polymer electrolytes because of the huge specific surface area of the nano-additive particles, or more precisely because of the numerous functional groups on the surface of the nanoparticles and the various components in the polymer electrolyte. Caused by the interaction.
2. The effect of nano Al2O3 particles on the ion conductivity of polymer electrolytes

Nano-scale (porous) additives have two important characteristics: they have a large specific surface area and are coated with various Lewis acidic or basic groups. The increase in conductivity is related to the interaction between the surface groups of the additives and the ions of the salt. Since the additives affect the physical and electrochemical properties of the electrolyte through the interaction of surface groups with the composite polymer electrolyte, from the perspective of basic research (that is, the goal is not to obtain the highest conductivity of the composite polymer electrolyte), nano ceramic additives The higher the mass fraction of the polymer electrolyte, the more obvious its influence on the properties of the polymer electrolyte system. Based on this consideration, the amount of filler used in this work is much higher than the amount used in practical polymer electrolytes.
Dissolve polyacrylonitrile (Mw=20000) and/or LiClO4 in an appropriate amount of propylene carbonate (PC), and add dry commercial Al2O3 (particle diameter 30nm) to the solution. The mixture was stirred and heated to dry at 120°C for 2 hours until most of the solvent was evaporated. Mix and grind this solvent-free mixture material with an appropriate amount of KBr, and place it in a vacuum oven (120°C, 0.1Pa) for 7 days after tableting. The actual measurement shows that the residual amount of solvent in the sample treated in this way is very low, and its influence on the infrared spectrum analysis of the sample can be ignored. Finally, the compressed tablets were sealed and packed in glass containers, and then transferred to the vacuum chamber of the Fourier transform infrared spectrometer for measurement before measurement. The resolution of the instrument was set to 2 cm-1.
(1) The influence of nano additives on the dissolution of lithium salt
The infrared spectrum of solid LiClO4 near 1120cm-1[v2(F2)] has multiple bands, with a peak at 966cm-1[v1(A1)], and also at 466cm-1 and 486cm-1[v2(E)]. All have peaks. The position of its characteristic peak v4 (F2) is particularly affected by the surrounding anion environment (according to the state of the salt, vibration peaks can appear at 624cm-1, 635cm-1 and 664cm-1). When the mass ratio of LiClO4/Al2O3 is 1:10, strong peaks can be observed at 627cm-1 and 636cm-1. The peak at 636 cm-1 is due to the presence of contact ion pairs, while the peak at 627 cm-1 is due to free anions that do not directly interact with Lit. The increase in ceramic powder content resulted in an increase in the intensity of the 627 cm-1 component and the attenuation of the 635 cm-1 component peak. This indicates that the presence of Al2O3 inhibits the association between Li+ and ClO4-, forming more free Li+ and ClO4-. Although the infrared absorption peak near 1120 cm-1 is very strong, it is not sensitive to the association of ions. In the polymer electrolyte, due to the action of Lewis acid, the dissolved Li+ will interact with the oxygen on the surface of α-Al2O3 particles, but due to the low content of nano-Al2O3 used, no change in peak intensity (I627/I634) is observed. Therefore, regardless of whether nano Al2O3 is added, PAN-based polymer electrolytes have the same solubility for LiClO4 salts, and as a result, the amount of Li+ dissolved by the polymer remains unchanged.
In the sample with a large amount of Al2O3, the peak at 634cm-1 is very weak, and the peak at 627cm-1 is dominant. This shows that as the content of additives increases, the bond between ClO4- and Li+ becomes weaker, and most of the ClO4- becomes free ions. In other words, adding Al2O3 to LiClO4 promotes the dissociation of solid LiClO4. In this sense, the nano-ceramic powder additive acts like a solid solvent in the LiClO4/Al2O3 composite, as shown in Figure 2.

Figure 2 The effect of nano-Al2O3 in iClO4/Al2O3 electrolyte on the decomposition of iClO4
(2) The effect of additives on polymer salt associations
There is a strong interaction between the cyano group (C≡N) of PAN and Li+. In pure PAN, the stretching vibration peak of the C≡N group is located at 2240cm-1. When C≡N…Li+ interaction occurs, a new peak appears at 2270cm-1. Figure 3 respectively shows the influence of the content of nano-ceramic additives [Figure 3(a)] and the content of salt [Figure 3(b)] on the interaction of polymer salt. When the salt content is very high or the additive content is very low, strong C≡N…Li+ interactions occur between the salt and the polymer. With the increase of ceramic powder content, the C≡N…Li+ interaction becomes insignificant or even disappears. These results indicate that the addition of nano-ceramic powder in the electrolyte weakens the interaction between C=N and Li+. At the same time, the addition of ceramic powder weakens the interaction between anions and cations, increasing the content of free anions.

Figure 3 The effect of Al2O3 in PAN/LiClO4/Al2O3 on the decomposition of R-C=N...Li+ complex
The above findings can be attributed to the possible competition between nano-ceramic particles and the interaction between Li+ and PAN cyano groups.
The research results in the PAN/LiClO4 composite system containing MgO, SiO2 and TiO2 show that the addition of nano-ceramic additives has an important influence on the interaction between the polymer electrolyte components and the internal microstructure. Because the particle size of the alumina is very small, it provides a large surface with many surface groups. For the interaction between the polymer and the salt in the composite, the properties of the surface groups of the ceramic particles are better than the chemical properties of the particles themselves. The ingredients are more important. As pointed out by Wieczorek and Croce et al., in nano-polymer electrolytes added with Al2O3 particles, the increase in conductivity depends on the properties of the surface groups of the filler.
The Lewis acid-base interaction theory of different kinds of surface groups can be used to fully explain this phenomenon. Jayathilaka et al. attributed the increase in conductivity in the (PEO)9/LiTFSI/Al2O3 system to a new path created by the interaction between the Lewis acid of the surface group of the nano-ceramic additive and the anion and cation of the salt. In acidic nano-additives, the strong attraction of anions to OH groups or the short-lived weak bond between Li+ and oxygen in basic additives forms a momentary OH…X- or O…Li+ bond. The instantaneous formation and breaking of these bonds provide additional locations for ions (carriers). This is somewhat similar to the ethylene oxide that cations migrate to PEO through intermittent association. Obviously, such a model only considers the interaction between the surface groups of the ceramic particles and the ions of the salt, while ignoring the influence of the additives on the interaction between the carrier and the polymer and the effect of the additives on the salt. , The role played by the association/dissociation of cations.
The electrochemical, mechanical and electrolyte/electrode interface properties of PAN nanopolymer electrolyte are similar to those of PEO-based electrolyte. According to the NMR measurement results of 7Li, there are three possible forms of lithium ions in polymer (gel) electrolytes containing plasticizers: ions that move quickly in the gel (similar to liquid) state, and in the solid PAN Slow-moving ions and ions that have a chemical shift in NMR due to association with plasticizers (solvents). Obviously, the freely moving Li+ in the gel state is the most important contributor to the conductivity of the polymer electrolyte. In actual polymer electrolytes, the formation of lithium ions that move slowly due to the association with the cyano group of PAN should be avoided as much as possible. Only when the salt content is high, ion clusters related or unrelated to PAN can make a significant contribution to the overall conductivity. At this time, the lithium ion transport mechanism changes from a general salt in polymer to a new polymer in salt. . This is completely different from PEO-based polymer electrolytes. In the PEO-based polymer electrolyte, since the segment movement of ethylene oxide is the main contributor to ionic conductivity, it is necessary to try to promote the interaction between Li+—O. In addition, in general salt in polymer electrolytes, efforts should also be made to avoid the formation of ion pairs and ion clusters. Therefore, the interaction mechanism proposed by Jayathilaka et al. and Croce et al. cannot explain the increase in ionic conductivity of PAN-based polymer electrolytes containing nano-ceramic additives.
In PAN-based electrolytes with or without plasticizers (solvents), when the lithium salt content is high, violent associations (such as RC≡N…Li+ and Li+ClO4-) will occur. Figure 4(a). Since the migration of Li+ associated with the cyano group must be achieved through the movement of the PAN segment, their mobility is very low, and their contribution to the ionic conductivity is also small. As Wieczorek et al. believed, for the PEG/LiClO4/Al2O3 system, when the acidic nanoceramic Al2O3 is added, the anions are more attractive to the acid radicals on the Al2O3 surface than the cations. Due to the stronger polarization of ClO4-, there is a stronger attraction between ClO4- and Al2O3 surface acid radicals. This will cause the dissociation of the Li+ClO4-ion pair to form free Li+. At the same time, since the polarizing ability of H+ to acidic groups is stronger than that of lithium ion to the cyano group of PAN, Li+ associated with the cyano group becomes a free ion. Such interaction increases the number of free Li+ in the polymer electrolyte, and therefore increases the ionic conductivity of the composite electrolyte. In addition, because the anions are captured by the oxygen atoms on the surface of the nanoparticles, the migration number of Li+ in such polymer electrolytes is also higher than when nanoceramic additives with other types of surface groups are used.

Figure 4 The surface of nano-Al2O3 particles (a) Lewis acidity; (b) Lewis basicity and (c) the effect of neutral groups on the dissociation of lithium salt
For Al2O3 with Lewis basic surface groups, the polar O atoms on the surface interact with Li+ and dissociate the Li+ClO4-ion pair [Figure 4(b)]. On the other hand, the strong interaction between O and Li+ breaks the R-C≡N…Li+ bond and frees ClO4-. When they migrate under an applied voltage, Lit dissociated from the salt interacts with polar oxygen atoms through instant hydrogen bonds. The cation can only temporarily maintain a hydrogen bond with oxygen. So there will be additional carrier migration near the additive particles. When the content of ceramic oxide is quite high [generally 10% to 20% (mass fraction) in the actual polymer electrolyte], this intermittent coordination provides a standard for carrier transport. Continuous effective channel. The interaction of basic surface groups with Lit or other positively charged ternary ion groups limits the interaction between salt and electrolyte. By studying the other two groups of electrolytes, the competition between the acidic or neutral surface groups of the additives and the Li+ in the polyether oxides was observed.
For neutral Al2O3 additives, both of the above-mentioned interactions will occur, so a higher concentration of carriers can be expected [Figure 4(c)]. But because the anion and Li+ re-associate to form a new association, the actual carrier concentration is lower than the above two.
Although the mechanism of action between inorganic nanoparticles and polymers needs to be further studied, due to the good mechanical properties of the nanocomposite polymer electrolyte, it does not contain a liquid all-solid structure, has high electrical conductivity and good interface stability. As well as electrochemical stability, PEO/LiX nanocomposite electrolyte becomes a promising electrolyte in polymer lithium ion batteries.