main content:
1. Types and composition of disposable batteries
A primary battery refers to a battery that cannot be used to restore the active material by a simple charging method after the battery is discharged.
As early as 200 years ago, the Italian Voda invented the first practical liquid battery. In 1836, Daniel invented the copper-zinc two-liquid battery. In 1863, the Leland Society invented the manganese battery, which made the dry battery, which is convenient for carrying, storage and use, replaced the wet battery and became the main category of primary batteries.
There are many types of disposable dry batteries (hereinafter referred to as dry batteries), but there are only a few series that are commonly used and used in large quantities.
2. Zinc manganese dry battery
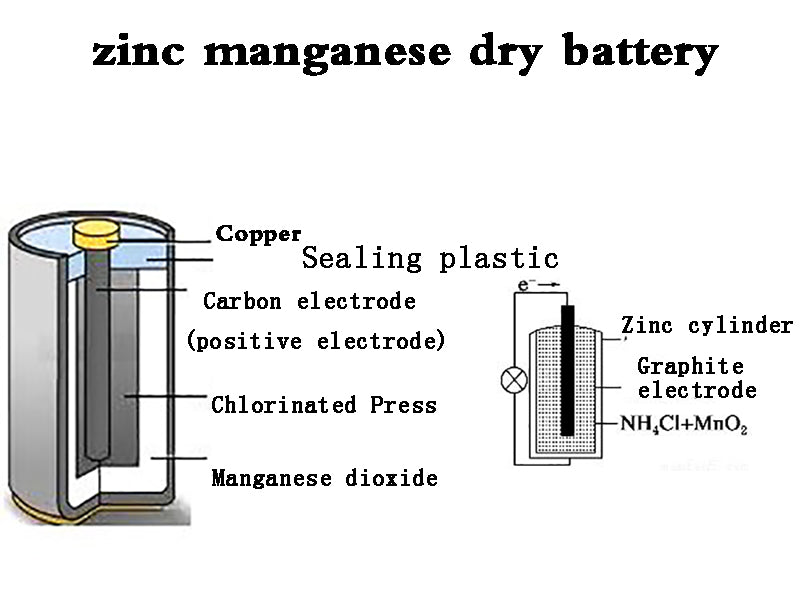
With manganese dioxide as the positive electrode, zinc as the negative electrode, and a chlorinated aqueous solution as the main electrolyte, paper, cotton or starch is used to gel the electrolyte so that it does not flow out. A battery with such a structure is called a manganese dry battery. This kind of battery was designed by the French Leland Society in 1868, so it is called the Lan Society battery in academia. The battery invented by Leland Society was not a dry battery, but a wet battery, which was later improved and mass-produced in 1890. In order to adapt to different purposes, one or several cylindrical, square, flat, button-shaped and other standardized single cells can be connected in parallel or in series to form a commercial battery.
The structure of a single cell is roughly divided into two types: simple (cylindrical and square) and flat. Among them, the output of cylindrical cells in the simple form accounts for the vast majority. A structure in which flat single cells are stacked and connected in series is called a stacked dry battery.
There are 26 types of cylindrical cells that have been standardized (International Electrotechnical Commission standard [EC86-2 (1963)]), but the widely used cylindrical cells are the Japanese names UM-1 (single), UM-2 ( Single 2) and UM-3 (single 3) for pen torches and other representative products, among which the most popular is the UM-1 type iron-shell battery.
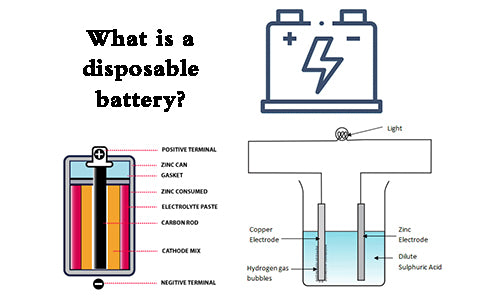
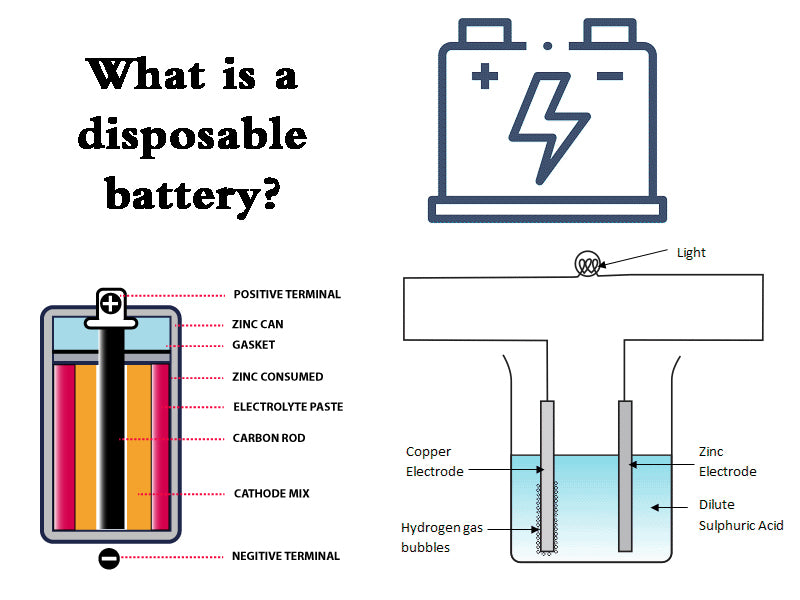
There is a carbon rod as a positive electrode in the center of the battery. This carbon rod is porous and has the effect of exhausting the gas generated during the discharge of the battery. Closely attached around the carbon rod is the positive electrode depolarizer, which is the so-called battery cell. The positive electrode depolarizer is a mixture of manganese dioxide, conductive carbon powder (acetylene black, graphite) and ammonium chloride powder with electrolyte (NH4Cl and ZnCl2 as the main components) and solidified to form. Between the positive electrode and the negative electrode zinc cylinder that doubles as a container, a gelatinized substance gelatinized with an electrolyte solution containing ammonium chloride and zinc chloride as the main components is contained with corn starch and wheat flour. An air chamber is left on the upper part of the cell as a space for exhaust gas and expansion of the cell. In order to prevent the evaporation of moisture, the upper part of the air chamber is sealed with a sealant. The top of the carbon rod has a metal metal cap as the positive terminal, and the zinc plate on the bottom of the negative zinc cylinder is the negative terminal. Due to excessive discharge consumption of the zinc cylinder, corrosion and perforation will occur, resulting in leakage. In order to prevent this leakage phenomenon, wrap paper or plastic tube on the outside of the zinc tube, and further use a metal tube for outer packaging. The positive and negative poles are in an insulating state, and both ends of the metal cylinder are bent inward and closed to form a packaging structure.
The structure of the Leranshe dry cell can be expressed as
(-)Zn | NH4Cl+ZnCl2 mixed solution (starch gelatinization) | MnO2+C(+)
The discharge mechanism of the battery is very complicated. First, the following reaction occurs on the negative electrode Zn (zinc amalgam).
Zn+2Cl-→ZnCl2 water+2e-
The electrode reaction of the positive electrode is
MnO2+e-→MnOOH
Its overall reaction formula is
Zn+2NH4Cl+2MnO2→Zn(NH3)2Cl2↓+2MnOOH
3. Alkaline zinc manganese dry batteries
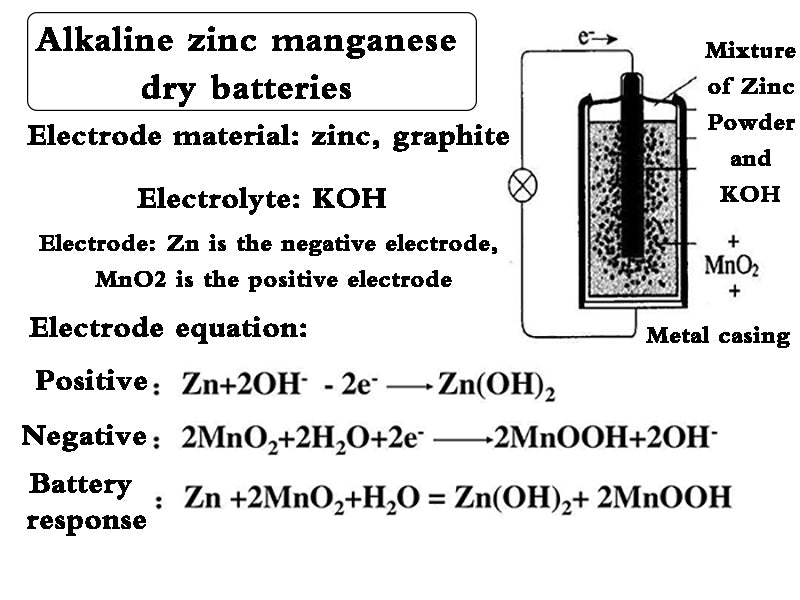
Starting with the commercialization of the flat alkaline manganese battery called "Crown" battery invented by WS Herbert of Yuehua Company in 1947, Mallory and Union Carbide Company in the United States have successively sold cylindrical batteries. Alkaline zinc-manganese batteries of the same size as Lanshe dry batteries. Because such batteries are particularly suitable for high-load discharge applications such as rotating machines, the demand for such batteries has increased dramatically in recent years. In Japan, Toshiba Yuehua, National Mallory, Hitachi Maxell, Matsushita Electric, Fuji Electric and other companies produce this kind of battery (code-named AM). Since the internal resistance of the battery is smaller than that of the Leranshe dry cell, and the internal resistance changes little during discharge, the battery has a high discharge voltage and a relatively flat discharge characteristic curve. Because of this, the alkaline zinc-manganese dry battery has the characteristics of small difference in duration between continuous discharge and intermittent discharge, and is especially suitable for high-load discharge. If the conditions of use are appropriate, the output power of alkaline zinc-manganese batteries is about 10 times that of Leland's dry batteries. In addition, alkaline zinc-manganese batteries can also be made into rechargeable batteries.
Alkaline zinc-manganese dry batteries are available in two types: cylindrical and flat. The latter has been produced in very small quantities. The standardized AMF battery has a similar structure to the flat mercury battery, the main use of which is for photographic flashes.
Commercially available cylindrical alkaline zinc-manganese batteries include AM-1, AM-2, AM-3, AM-4, AM-5, AM-5h of the same model and size as zinc-manganese dry batteries (considering The interchangeability of the battery HR, but the reverse-polar structure is adopted, that is, the head is the negative electrode) six kinds.
The negative electrode zinc powder (amalgamated, the mercury content is about 3%~13%, and the particle size of the zinc powder is different in each factory) is formed into a cylindrical shape and placed in the central part of the battery; or CMC (methyl cellulose) and so on to make the powder colloidally dispersed in the electrolyte. The cylindrical fibrous electrolyte separator containing caustic bell (in about 30% KOH, adding different amounts of ZnO according to the situation) is installed in a steel casing (Ni or Au, etc.) in close contact with the inside of the shell. inside the ring cell. The outer shell of this kind of battery is the positive electrode, and the zinc in the central part is the negative electrode, which constitutes a so-called reverse pole structure.
The composition of the battery is as follows.
(-) Zn (ammalgamation) | NaOH or KOH (30%~40%) aqueous solution + ZnO | MnO2 + graphite (+)
The positive electrode reaction of the alkaline zinc-manganese battery is roughly the same as that of the Leranshe zinc-manganese dry battery, which is simply described as
MnO2+e-→MnOOH
The negative electrode reaction can be expressed as a whole by the following formula.
Zn+2OH-→ZnO+H2O+2e-
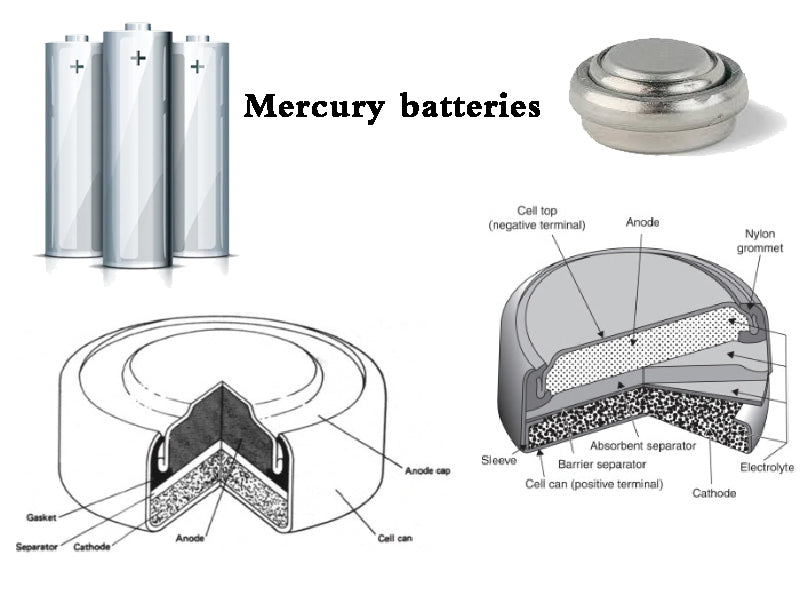
4. Mercury batteries
Due to the rapid development of electronic instruments, Rubin and Mallory have jointly created a battery with mercury oxide (HgO) as the positive active material (also known as RM battery), the composition of the battery is as follows.
(-)Zn(Hg)|KOH(ZnO) aqueous solution|HgO(+)
This battery was reserved for military use during World War II. At present, it is used as a power supply for small electronic instruments, and is used in various household appliances such as hearing aids, cameras, watches, medical instruments, etc. Mercury batteries have 3 to 4 times the discharge capacity than zinc-manganese dry batteries of the same size. However, due to the high price of mercury oxide, the economic performance of mercury batteries is poor.
Mercury batteries generally have two types: cylindrical and flat. No matter which one is sealed with a steel container, the positive electrode is a cell formed by HgO and graphite pressure molding, which is closely connected to the nickel-plated steel cylinder; the negative electrode is zinc powder (amalgam), and the surface in contact with zinc is usually It is a steel rod plated with Sn or Cu, which can prevent the generation of hydrogen due to local action. Larger batteries use two-layer steel cylinders. When a small amount of gas is accumulated inside, due to the action of internal pressure, it will pass through the sealing ring of synthetic rubber or plastic sandwiched between the two layers of steel cylinders and be discharged out of the cylinder from the gap between the two layers of cylinders. In addition, the paper cylinder is placed between the two layers of steel cylinders to absorb the electrolyte or moisture brought out when the gas is discharged, so as to prevent liquid leakage to the outside. The problem that should be paid attention to in the structure is that the polarity of the cylindrical mercury battery is opposite to that of the general dry battery, that is, the sealing cap is the negative electrode, and the container is the positive electrode.
The negative electrode of the small flat mercury battery is mostly formed by granular zinc; the negative electrode of the cylindrical mercury battery is mostly formed by the paste colloid of granular zinc and CMC-Na. Regardless of the form of the electrode, try to expand the surface area of the zinc electrode in order to improve the utilization rate of zinc. The amalgamation of Zn surface with Hg can improve the discharge performance and storage performance of the battery.
The positive active material is mercury oxide (HgO), which is added with 5% to 10% of graphite as a conductive agent. After mixing, it is directly pressed into the battery container under high pressure;
The battery capacity is calculated as about 0.2A•h/g according to the mass of the cell powder. When the battery is discharged, almost all of the active materials are used, so the amount of oxidation can be calculated according to the amount of zinc in electrochemical equivalents, that is, the capacity of 1A h consumes 1.220g of zinc and 4.041g of mercury oxide. Batteries combined according to this mass ratio are called trim batteries. After the battery is fully discharged, all Zn is oxidized, so usually the parts in contact with Zn should use metal sealing caps such as Cu-plated or Sn-plated steel plates (amalgamated). The HgO in the positive electrode turns into Hg after discharge, and gradually aggregates into a spherical shape, which often causes a short circuit between the positive and negative electrodes. In order to prevent this from happening, a separator that can hold the electrolyte is used between the positive and negative electrodes. The separator is an alkali-resistant felt-like fiber cloth, special cellophane or plastic separator paper. The use of this isolation layer prevents short circuits caused by discharge products [such as Hg and ZnO (semiconductor)] and graphite between the electrodes.
The electrolyte is an aqueous solution of saturated caustic alkali with ZnO added (for example, using a 43% aqueous KOH solution with a relative density of 1.53; or using a 30% aqueous NaOH solution with a relative density of 1.42).
The composition of the battery is as follows.
(—) Zn (Hg 10%) | NaOH (30%) or KOH (43%) aqueous solution with ZnO, saturated | HgO (MnO2) + C (+)
On the positive electrode, mercury oxide undergoes a reduction reaction to form mercury.
HgO+H2O+2e-→Hg+2OH-
The total reaction of the negative electrode is then
Zn+2OH-→ZnO+H2O+2e-
5. Silver oxide battery
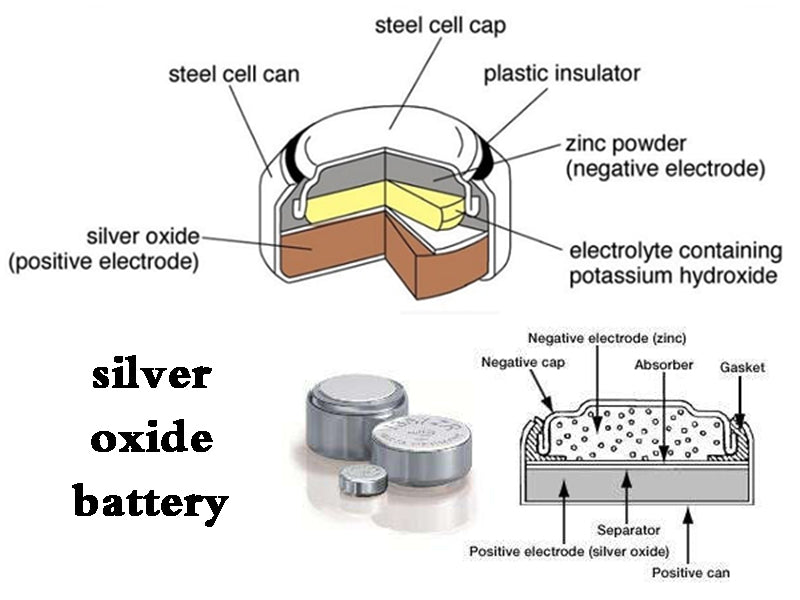
The sealed small primary battery composed of silver oxide and zinc has a very high specific energy and a very stable discharge voltage, which is one of the high-performance batteries. The battery was put on the market around 1963. Since the positive electrode active material uses a silver compound, the cost of the battery is relatively high, and now it is limited to the production of small products of 30~150mA•h. Mainly used for the power supply of battery-operated watches, hearing aids, cameras, ultra-small measuring instruments, etc.
The positive electrode uses silver oxide (Ag2O), the negative electrode uses zinc powder, and the electrolyte uses caustic potash or caustic soda. The discharge reaction is carried out as follows.
Positive electrode: Ag2O+H2O+2e-→2Ag+2OH-
Negative electrode: Zn+2OH-→ZnO·H2O+2e-
The total battery reaction is: Ag2O+Zn→2Ag+ZnO
The conductive material graphite is mixed into the positive electrode silver oxide. In order to slow down the sharp drop in the voltage at the end of the discharge, a part of manganese dioxide is sometimes added. In order to prevent the corrosion of the zinc powder used in the negative electrode, amalgamation treatment should be carried out, and alkali-resistant paste and electrolyte should be added to make it into a gel before use. For example, if the watch uses a battery that is used for a long time at a small current, caustic soda electrolyte can be used; if the battery is used at low temperature or high current, caustic potassium electrolyte can be used. Since silver oxide is slightly dissolved in the electrolyte (about 5×10-4mol/L Ag+), once the dissolved Ag+ ions diffuse to the zinc electrode, it will cause local action or cause short circuit between electrodes. Due to measures such as reducing the amount of electrolyte in this battery and using special separators (such as cellophane, polyvinyl alcohol films and other semi-permeable membranes) to prevent the diffusion of Ag+ ions, the battery has almost no use in use. Problems (more than 1 year of storage can still maintain more than 90% of the remaining capacity).