
main content:
A battery is a power source that obtains energy through electrochemical reactions. In 1780, Italian physicist Alessandro Volta prepared the first demonstration application device to obtain electricity through electrochemical reaction. However, the first battery was made in 1866 by Georges Leclanché. There are two types of batteries on the market: primary batteries (batteries that can only be used once) and secondary batteries (batteries that are rechargeable and reused many times). At present, battery products are widely used in many professional and non-professional applications, such as machine tools, instruments, medical devices, safety devices, telephones, toys and video recorders.
1. Battery category

In addition to a wide variety of chemistries, batteries can vary widely in size, from button cells used in medical applications to large multi-compartment batteries used with load level measurement systems.
Primary batteries are all for small devices. A primary battery consists of irreversible electrochemical electrode pairs that can no longer be recharged and must be replaced with a new battery when the power is exhausted. Therefore, people keep buying and using primary battery products, and keep discarding used primary batteries. These wastes are often found in municipal solid waste.
Secondary batteries are used in a wide range of applications, ranging from commonly used cellular phones to heavy-duty level measuring electrical devices. The use of reversible electrochemical electrodes allows the secondary battery to be recharged. In the manufacture of rechargeable batteries, the following three metals are mainly used: lead, bromine and lithium. The flow direction of the secondary battery at the end of its life depends on the type and life of the battery. Lead-acid batteries are mostly used for starting automobiles, and many factories are designated to recycle and utilize these waste lead-acid batteries. Lithium batteries are a new product on the market, generally speaking, lithium batteries are smaller. However, many batteries are currently discarded as municipal solid waste or placed in home backyards, with potential impact on the environment in the future.
In addition, various chemical substances are contained in secondary batteries. Secondary batteries vary widely in size and shape, from small accumulators to large multi-compartment safety battery systems.
The main types of batteries include ordinary zinc-manganese batteries, alkaline zinc-manganese batteries, Jin pot batteries, lead-acid batteries, Jin hydrogen batteries, lithium batteries, etc.
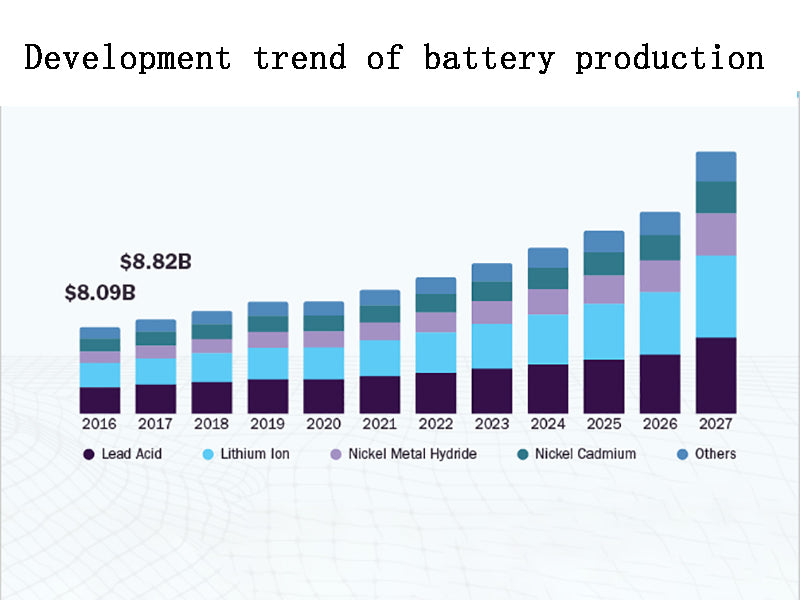
2. Development trend of battery production
In the relevant policies, regulations on the prevention and control of special hazardous waste pollution: the national and local governments at all levels should formulate technical and economic policies to eliminate batteries containing mercury and cadmium; manufacturers should adjust the product structure in accordance with national laws and industrial policies of various countries, and eliminate the batteries containing mercury and cadmium on schedule. Mercury and cadmium batteries; before the batteries containing mercury and cadmium are eliminated, the municipal solid waste treatment unit should establish classified collection, storage and treatment facilities to effectively manage waste batteries; promote the classified collection of waste batteries to avoid mercury, Cadmium waste batteries are mixed into domestic waste incineration facilities; waste lead-acid batteries must be recycled and not disposed of by other methods, and their collection and transportation must be included in hazardous waste management.
The focus of battery industry development and adjustment is mercury-free alkaline zinc-manganese batteries, Jin-hydrogen power batteries, lithium-ion batteries, fully sealed maintenance-free lead-acid batteries, fuel cells, solar cells and other new high-energy batteries.
The average annual growth of primary batteries is 200 million. Therefore, we should focus on adjusting the product structure, increasing the proportion of alkaline zinc-manganese batteries, and speeding up the process of low mercury and no recording. The production of mercury oxide batteries and paste batteries is strictly prohibited, and the further improvement of performance and mercury-free production of alkaline zinc-manganese batteries are encouraged; the production of mercury-free alkaline zinc-manganese batteries by electrolytic manganese dioxide and mercury-free zinc powder is encouraged. , and further improve the battery quality.
In small secondary batteries, Jin-cadmium batteries need to gradually reduce production and increase the proportion of mercury-free and cadmium-free batteries. Jin hydrogen battery is based on 80 million units, with an average annual growth rate of 45%. Based on 200 million lithium batteries, the average annual growth rate is 108%. The average annual growth rate of lead-acid batteries is 5%, and fully sealed maintenance-free lead-acid batteries will have an absolute advantage.
3. Main pollutants in waste batteries

The main pollutants contained in batteries include heavy metals and electrolyte solutions such as acids and alkalis. The main heavy metals are mercury, cadmium, lead, nickel, zinc and so on. Mercury, cadmium and lead are substances that are harmful to the environment and human health; nickel and zinc are beneficial substances within a certain concentration range, but will cause harm to the human body when they exceed a certain amount in the environment; waste acid and waste alkali Such electrolytes may acidify or alkalize the soil. Waste batteries containing waste acid, waste alkali and other components of waste electrolyte (such batteries are mainly lead-acid batteries and Jin-cadmium batteries for starting waste cars) may cause heavy metals to pollute the environment, and may also cause the electrolyte to cause environmental pollution. Pollution. This pollution is both immediate and long-term. Table 1 shows the main components of waste lead-acid batteries. The health hazards of the main metals contained in waste batteries are shown in Table 2.

Table 1 Composition (mass fraction) of waste lead-acid batteries Unit: %
| Element | Harm |
| HG | It is carcinogenic; it can cause central nervous system diseases, and it is the main culprit of Japan's "water conservation disease". Among them, organic mercury compounds (methyl mercury) are the most toxic, which can damage the basic functions and metabolism of cells, damage liver function, and cause kidney failure. |
| cadmium | Carcinogenic; mainly damages the kidneys and liver, followed by osteoporosis, cartilage, and fractures, the so-called "bone pain disease" |
| Nickel | It is carcinogenic; it has obvious harm to aquatic organisms. The unique symptoms of nickel poisoning are dermatitis, respiratory disorder and respiratory cancer. |
| Zinc | The main poisoning symptoms are gastrointestinal dysfunction and diarrhea. Zinc salts can cause dermatitis and skin ulcers. Inhalation of zinc oxide fumes can cause zinc poisoning and produce metal fume fever symptoms, causing severe bronchitis and pneumonia. |
| Copper | Copper salts are highly toxic, can cause damage to the gastrointestinal system, can cause hemolytic anemia, disrupt bile excretion, and cause liver damage |
| Lead | The main toxic effect is to cause anemia, damage the nervous system, cardiovascular and kidneys, damage the digestive system, and affect the intellectual development of children |
Table 2 Harm of main metals in waste batteries to human body















