main content:
In addition to the reductive decomposition of the electrolyte that occurs under normal use, another major cause of electrolyte decomposition is overcharge of the battery. If a small part of the electrolyte is consumed during each charge, more electrolyte needs to be added when assembling the battery. The solid product generated by the decomposition of the electrolyte may form a passivation layer on the electrode, increase the polarization of the battery, and reduce the output voltage of the battery. The overcharge of the battery includes three aspects: the overcharge of the negative electrode, the positive electrode and the electrolyte.
1. Electrolyte reduction and decomposition caused by overcharge of the negative electrode
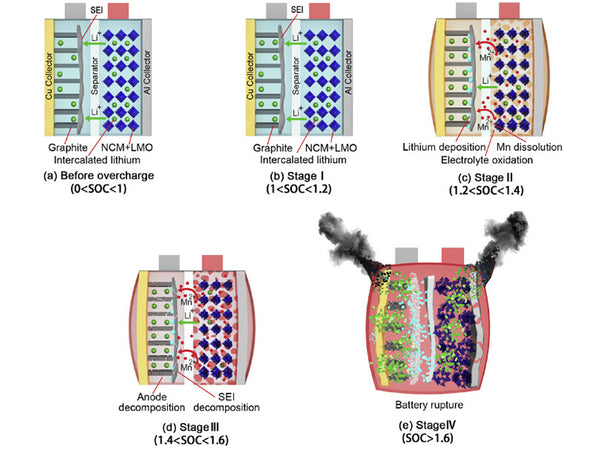
There are two situations that may cause overcharging of the battery. Overcharging causes the lithium ions of the positive electrode to be deposited on the surface of the negative electrode in the form of metallic lithium. The reason is that the battery contains too much positive electrode material and too little negative electrode material. Since there are not enough lithium-absorbing sites in the negative electrode material (formation of intercalation compounds or generation of lithium alloys), excess lithium can only be deposited on the surface of the negative electrode in the form of metallic lithium. Another situation occurs when the battery is discharged at a high rate. Due to the polarization of the negative electrode under high-rate discharge conditions, lithium ions are too late to diffuse into the graphite carbon layer to form intercalation compounds or form LixM alloys, and are reduced to metal on the surface of the negative electrode. lithium. Since the potential at the boundary between the electrode and the diaphragm is the most negative, lithium metal is most easily deposited at these locations. Due to the high activity of metallic lithium, the deposited lithium covering the surface of the negative electrode will quickly react with the solvent or salt, causing additional loss of the electrolyte, and simultaneously generating Li2CO3 and LiF.
The potential difference between metallic lithium and the lithium-inserted carbon electrode is very small. Therefore, the reductive decomposition of the electrolyte on the carbon electrode is very similar to that on the metallic lithium. Some physical research methods can be used to study the reduction and decomposition of the electrolyte on the negative electrode surface, such as X-ray photoelectron spectroscopy (XPS), Auger electron spectroscopy (AES), energy dispersive X-ray analysis (EDAX), Raman spectroscopy, in-situ and ex-situ Fourier transform infrared spectroscopy (FTIR), atomic force microscope (AFM) and electron spin resonance (ESR), etc.
During the first discharge, the electrochemical decomposition reaction of PC on the graphite electrode can be regarded as a two-electron reaction process. This process takes place near 0.8V, and the reaction products are mainly Li2CO3 and propylene, namely:
2Li++2e+(PC/EC)→Propylene (gas)/ethylene (gas)+Li2CO3 (solid)
ROCO2Li is produced through the single-electron reduction process of PC, such as CH2CH(OCO2Li)CH2OCO2Li, ROCO2Li reacts quickly to Li2CO3 when it encounters trace amounts of water.
When crown ether is present in the electrolyte, the solvent will be reduced on the surface of the carbon electrode and will not enter the interior of the carbon through co-intercalation. Therefore, the carbon electrode can maintain its graphite structure and undergo multiple reversible lithium intercalation reactions. When the reductive decomposition of PC only occurs on the surface of the carbon electrode, the charge transfer is mainly carried out through the SEI film. Due to the lack of driving force to reduce the PC, the single-electron reductive decomposition of PC is the most advantageous. When there is no crown ether in the electrolyte and only PC is used as the solvent, the PC co-intercalation occurs at the first discharge, the graphite structure is pulverized and destroyed, and the intercalation compound cannot be formed. Most of the reductive decomposition of PC occurs inside the carbon electrode. In this case, the two-electron reduction process is conducive to the formation of Li2CO3.
The capacity loss in the first cycle may be caused by the decomposition of PC to produce Li2CO3. In addition, there are other side reactions. During the first charge, the decomposition of PC may occur through two paths, one is that PC is directly reduced to produce propylene and Li2CO3; the other is that PC is first reduced to fragment anions, and then through the connection of fragment endpoints to form alkyl ester lithium . These lithium alkyl esters are unstable, and they are reduced to produce also unstable fragment compounds, and react with propylene to form oligomer fragments, and finally are oxidized to compounds containing C-H bonds and COOH groups.
The following summarizes the decomposition mechanism of some common electrolyte solvents and lithium salts.
① Decomposition of PC. The two-electron reduction mechanism proposed by some people is:
PC+2e→Propylene+CO32-
The single-electron reduction mechanism proposed by Aurbach et al. is:
PC+e→PC- (fragment anion)
2PC-+e→Propylene+CH3CJ(OCO2)-CH2(OCO2)-
CH3CH(CO3)-CH2(CO3)-+2Li+→CH3CH(OCO2Li)CH2OCO2Li (solid)
② The decomposition of EC, the two-electron reduction process of EC is similar to the two-electron reduction process of PC:
EC+2e→ethylene+CO32-
The single-electron reduction mechanism of EC is also similar to the one-electron reduction process of PC:
EC+e→EC-(fragment anion)
2EC-+e→Ethylene+CH3CJ(OCO2)-CH2(OCO2)-
CH3(OCO2)-CH2(OCO2)-+2Li+→CH3(OCO2Li)CH2OCO2Li (solid)
The EC decomposition product CH3 (OCO2Li) CH2OCO2Li can be used as an effective passivation film, which is similar to the effect of Li2CO3.
③ Decomposition of DMC. The decomposition of DMC can be written as :
CH3OCO2CH3+e+Li+→CH3OCO2Li+CH3*
Or CH3OCO2CH3+e+Li+→CH3OLi+CH3OCO*
Both CH3OLi and CH3OCO2Li are formed by a nucleophilic reaction on DMC. According to Aurbach et al., the generated fragments ( CH3*and CH3OCO*) are converted into CH3CH2OCH3 and CH3CH2OCO2CH3.
④ Decomposition of DEC. It can be written as:
CH3CH2OCO2CH3+2e+2Li+→CH3CH2OLi+CH3CH2OCO*
Or CH3CH2OCO2CH3+2e+2Li+→CH3CH2OCO2Li+CH3CH2*
According to Aurbach et al., the fragments (CH3CH2OCO* and CH3CH2* ) generated by the decomposition of DEC are converted into CH3CH2OCH2CH3 and CH3CH2OCO2CH2CH3.
⑤Reductive decomposition of several commonly used lithium salts. Since the reduction products of the salt also participate in the formation of the SEI film, the type and concentration of the electrolyte salt also have an important influence on the performance of the carbon insertion electrode. In some cases, the reduction of the salt may have an important effect on the stability of the electrode surface and the formation of the required passivation interface. In other cases, the salt decomposition deposits may affect the reduction products of the solvent. The reductive decomposition of the lithium salt LiCF3SO3 on the negative electrode occurs before the decomposition of the solvent (PC/EC/DMC). The reductive decomposition process of several lithium salts is as follows.
LiAsF6: LiAsF6+2e+2Li+→2LiF+AsF3
AsF3+2xe+2xLi+→LixAsF3-x+xLiF
LiClO4: LiClO4+8e+8Li+→4Li2O+LiCl
Or LiClO4+4e+4Li+→2Li2O+LiClO2
Or LiClO4+2e+2Li+→Li2O+LiClO3
LiPF6: LiPF6⇆LiF+PF5
PF5+H2O→2HF+PF3O
PF5+2xe+2xLi++H2O→xLiF+LixPF5-xO+2H+
PF6-+2e+3Li+→3LiF+PF3
The decomposition of LiBF4 is similar to that of LiPF6:
BF4-+xe+2xLi+→xLiF+LixBF4-x
Decomposition of LiBF4 in water:
Li++BF4-→LiF(solid)+BF3(gas)
BF3+H2O→2HF+BOF
Reduction of impurities. The electrolyte often contains various impurities, such as oxygen and water. After the oxygen is reduced, it combines with lithium to form lithium oxide.
O2+4e+4Li+→2Li2O (solid)
The trace amount (100~300μg/g) of water in the electrolyte generally does not affect the performance of the graphite electrode. When the water content is relatively high, in the presence of Li+, after the water is reduced (approximately 1.4V), it will react with Li+ to generate LiOH and deposit on the surface of the carbon electrode, which becomes a high-impedance interface layer, which hinders the insertion of lithium ions. In graphite:
2H2O+2e→2OH-+H2
Li++OH-→LiOH (solid)
2LiOH(solid)+2e+2Li+→2Li2O(solid)+H2
When CO2 is present, Li2CO3 is generated, which is deposited on the negative electrode as one of the components of the passivation layer.
2CO2+2e+2Li+→Li2CO3+CO
Or CO2+e+Li+→CO2-·Li+
CO2Li+CO2→OCOCO2Li
OCOCO2Li+e+Li+→CO+Li2CO3
Secondary reaction. Lithium carbonate can be formed through a secondary reaction:
2ROCO2Li+H2O→2ROH+CO2+Li2CO3
Wherein, R represents a vinyl group or a propylene group. LiAsF6 and LiPF6 are reduced when the potential with respect to metallic lithium is 1.5V.
2. Oxidation and decomposition of electrolyte caused by positive electrode overcharge
For batteries with different composition systems, overcharging of the positive electrode of a lithium-ion battery will also cause a series of electrochemical reactions. The reaction related to electrolyte decomposition is that transition metal oxides with low lithium content (such as LiyNiO2, when y<0.2) have a strong catalytic effect on the oxidation of electrolytes, especially solvents. The composition of the positive electrode material affects the decomposition of the electrolyte. In a lithium ion battery with artificial graphite as the negative electrode, when LiCoO2 is used as the positive electrode material and above 4.0V, the swelling of the battery is mainly caused by the reaction on the positive electrode, while below 4.0V, it is mainly caused by the negative electrode. Caused by the reaction on the site. Analysis of the gas composition and the dependence of gas swelling on voltage shows that gas swelling is caused by the oxidation of the electrolyte on the positive electrode during the charging process and the reduction of lithium from the graphite during the discharge process. When LiNi0.8Mn0.1Co0.1O2 is used as the positive electrode, the electrolyte will oxidize and swell on the positive electrode material above 3.2V, and the swelling phenomenon on the LiNi0.8Mn0.1Co0.1O2 positive electrode is far greater than that on the negative electrode. Much more serious.
3. Oxidation and decomposition of electrolyte at high charging voltage
Most electrolytes will decompose at high charging voltages (>4.5V) to generate insoluble products such as Li2CO3, which block the micropores on the electrode and generate gas. This will not only cause battery capacity loss during the cycle, but also cause serious safety hazards.
The oxidation process of the solvent can generally be described as:
Solvent→oxidation product (gas, liquid and/or solid species) +ne
The oxidation of the solvent will cause a decrease in the content of the solvent, the salt content in the electrolyte will increase relatively, the conductivity of the electrolyte will decrease, the polarization of the battery will increase, and the average output voltage of the battery and the actual achievable capacity will decrease. At the same time, the accumulation of gas products and other species generated by the oxidation and decomposition of the solvent in the battery will also cause a series of problems. The rate of oxidative decomposition of the solvent is determined by the surface area of the positive electrode material, the current collector material used, and the properties of the conductive additives. In fact, due to the huge specific surface area of carbon black materials, the type of carbon black additives and the selection of specific surface areas are extremely critical. Solvent oxidation is likely to occur more on the carbon black conductive agent rather than metal oxidation. On the object electrode.
The decomposition voltage of the electrolyte is usually characterized by cyclic voltammetry, and the electrode used can be an inert metal or an insert electrode material actually used in the battery. For irreversible electrochemical side reactions, since there is no thermodynamic open circuit voltage, the decomposition voltage itself does not have much practical significance. Instead, these side reactions can be described by the Tafel equation. At any potential, a finite decomposition rate will be obtained according to the Tafel equation, which increases with the increase of the voltage.
Studies have found that when the potential for lithium is as low as 2.1V, PC will be oxidized and decomposed. Above 3.5V, the oxidative decomposition rate of PC will increase significantly. Depending on the electrode material, the oxidative decomposition of PC may start at 2V. However, in actual research, it is often seen that the oxidation potential of PC is much higher than 2V (usually above 4.5V), and the decomposition of LiClO4/PC and LiAsF6/PC electrolyte on the heat-treated MnO2 electrode occurs at 4.0V However, at lower potentials (LiClO4/PC is 3.15V, LiAsF6/PC is 3.4V), CO2 is already generated. No CO2 production was observed during the reverse (cathode) scan.
Above 4.5V, ClO4- decomposes to form chlorides such as ClO2 and HCl. The decomposition mechanism may look like this:
ClO4-→e+ClO4→ClO2+2Oad+e
ClO2+H++e→HCl+O2(g)
2Oad→O2(g)
Using differential electrochemical mass spectrometry, it can be known that the oxidation potential of ClO4- on the Pt electrode is 4.6V. Due to the instability of ClO2, it will generate HCl in an environment that may cause the protons produced by the oxidation of PC.
LiPF6 is currently the most widely used electrolyte salt. Due to the synthesis process (PF5+LiF→LiPF6), the Lewis acidity of PF5 will cause ring-opening polymerization of EC molecules in the electrolyte. Below 170°C, this polymerization reaction is an endothermic reaction. Due to the CO2 change from the oligoether carbonate polymer, it will produce a polymer similar to PEO. CO2 is present in the heated electrolyte solution. Above 170°C, the polymerization reaction becomes exothermic and causes violent electrolyte decomposition. In actual batteries, PEO-like polymers will further react with PF5 to form electrolyte-soluble products or participate in the formation of SEI membranes. GPC analysis of the heated electrolyte shows that species with a relative molecular mass (Mw) of 5000 exist in the product. Transesterification and polymer species have been observed in the electrolytes of cycled and aged lithium-ion batteries. After cross-linking with acidic species, the resulting polymer product will cause the degradation of the electrolyte transport properties in the composite electrode and the attenuation of the power and energy of the battery. In a battery with good sealing properties, the CO2 generated due to the thermal decomposition of the solvent saturates the electrolyte and even terminates the polymerization reaction. CO2 is easily reduced on the negative electrode to produce oxalate, carbonate and CO. The resulting carbonate helps to form the SEI film. However, oxalate is easily dissolved and re-oxidized on the positive electrode to form CO2, which is a reversible self-discharge reaction. If CO2 is irreversibly reduced to carbonate and CO, it will cause irreversible self-discharge.
Someone has studied the ring-opening reaction of PC on Pt, Al, Au and Ni electrodes, and the results show that in different PC-based electrolytes, the anode behavior of Ni electrodes depends on the electrolyte salt used. At high electrode potentials, the decomposition products depend on the anion species of the salt. Cyclic voltammetry has also experimentally confirmed the oxidation of the electrolyte during overcharge of lithium-ion batteries, but these studies have not explained the mechanism of electrolyte decomposition or the analysis of the types of reaction products.
During the first cycle, the electrolyte decomposition is accompanied by gas production and battery swelling. The gas generation reaction on the carbon electrode is caused by the decomposition of the electrode, the reduction of reactive trace impurities and the electrolyte. Among them, the solvent decomposition in the electrolyte is the main reason for the first capacity loss. When using EC/DMC and EC/DEC 1mol/L LiPF6 electrolyte, the gases produced include CO2, CO, CH4, C2H4, C2H6, etc. Li2CO3, ROCO2Li, (ROCO2Li)2 and RCO2Li are formed on the surface of the carbon electrode. After adding 0.05mol/L Li2CO3 as an additive to the electrolyte, the total volume of gas generated decreases, the lithium ion conductivity of the electrolyte and the discharge capacity of the battery increase. This is because the thin and dense solid electrolyte membrane formed by Li2CO3 on the carbon electrode effectively prevents the co-intercalation of the solvent and the delamination of carbon. Using radio frequency sputtering to deposit a solid electrolyte film lithium phosphorus oxide nitrogen (LiPON) on the surface of the carbon electrode can also inhibit the solvent decomposition on the carbon electrode and reduce the irreversible capacity loss in the first cycle.
The decomposition of the electrolyte is closely related to the composition of the electrolyte. For example, after 1500 cycles of a carbon/LiCoO2 battery system using 1mol/L LiPF6-PC/EMC/DEC/DMC as the electrolyte at 4.2~2.5V, a small amount of methane gas produced by the decomposition of DMC is detected. Only under the conditions of overcharge and overdischarge, solvent molecules (PC, EMC, DEC and DMC) will be significantly decomposed, releasing CO2, CH4, C2H6, C3H6 and other hydrocarbons. Since these generated gases will increase the internal pressure of the battery and are mostly flammable, reducing the decomposition reaction of the electrolyte and the capacity loss to a minimum is the need to extend the cycle life of the battery and improve the high-temperature performance of the battery.
The application of density functional theory to study the reduction and decomposition of EC molecules in the electrolyte solution found that although the reduction of EC in the gas phase is thermodynamically forbidden, in the condensed solvent, EC may undergo a single-electron or two-electron reduction process. Decomposition, and the presence of lithium ions in the solution makes the intermediate product of the EC reduction reaction more stable. As the number of EC molecules increases, the adiabatic electron affinity of supermolecule Li+(EC)n (n=1~4) gradually decreases, regardless of the reduction of EC or Li+. In the process of reductive decomposition, Li+(EC)n is first reduced to an ion-pair intermediate. After passing through a potential barrier of approximately 46.024kJ/mol (11.0kcal/mol), the intermediate will then break the homogeneously split CO bond, generating fragment anions and Lithium ions associate. Among the possible termination paths of the fragment anion, the thermodynamically most favorable path is the formation of dicarbonate lithium butene (CH2CH2OCO2Li)2, followed by the formation of an O-Li bond compound containing an ester functional group [LiO(CH2)2CO2]2, Then there are two very competitive reactions: the further reduction of fragment anions and the formation of ethylene biscarbonate lithium (CH2OCO2Li)2. The most unfavorable reaction is the formation of Li carbide Li(CH2)2OCO2Li. As shown by the reaction between LiCO3- and Li+(EC)n, the product has little dependence on EC concentration. Under the condition of low EC concentration, the formation of Li2CO3 is slightly dominant. Only when the EC concentration is high, is it beneficial to produce (CH2OCO2Li)2. According to some calculation results and combined with experiments, it is believed that the two-electron reduction process does occur through a gradual path. Most experimental results have shown that (CH2OCO2Li)2 is the main component of the surface film produced by solvent reduction. The surface film is mainly composed of two kinds of alkyl biscarbonate lithium (CH2CH2OCO2Li)2 and (CH2OCO2Li)2, in addition to Li(CH2)2OCO2Li, Li2CO3 and LiO(CH2)2CO2(CH2)2OCO2Li. Using the rotating ring disk electrode system and ab initio molecular orbital calculations to study the initial reaction in the reductive decomposition of lithium battery electrolyte solvents, it is proved that the electron transfer is induced by cathodic polarization, and the formed soluble reduction products can be oxidized again. The electron transfer process from the electrode to the solvent molecule associated with the lithium ion is an exothermic reaction with a reaction energy of -3eV. This is the initial reaction of electrolyte reduction and decomposition and must be controlled to improve the performance of lithium-ion batteries and lithium batteries.
















