
With the rapid popularity of lithium-ion batteries for civilian use and the large-scale application of electric vehicles in the future, battery safety issues have become increasingly prominent. Organic electrolytes are extremely flammable substances. Overheating and overcharging and discharging of the battery may cause the burning of the electrolyte or even the explosion of the battery. Therefore, the battery overcharging problem must be considered when designing the battery, and appropriate measures should be taken to prevent the battery from overcharging.
Overcharging will not only cause a series of side reactions in the positive and negative electrodes and electrolyte of the lithium-ion battery, resulting in the loss of active materials and electrolyte consumption, resulting in the loss of battery capacity, and more likely to cause safety issues. The internal temperature of the battery will increase significantly during charging and use. The overcharge and overdischarge of the battery will cause the violent decomposition of the electrolyte, and at the same time, accompanied by the generation of a large amount of heat and gas, the internal temperature and pressure of the battery will increase. Therefore, the safety protection of the battery is mainly considered from the three aspects of battery voltage, internal temperature and internal air pressure. From the perspective of electrolyte, the safety measures taken can be divided into two aspects: inside the electrolyte and outside the electrolyte. At present, commercial lithium-ion batteries generally have taken safety protection measures in addition to the electrolyte, including the following aspects.
①Explosion-proof valve. When the internal pressure of the battery increases abnormally, the explosion-proof valve deforms and cuts off the lead wire placed inside the battery for connection, and the battery stops charging.
② Diaphragm with self-closing mechanism. The polymer separator of a liquid lithium ion battery is usually a porous membrane.
Under normal circumstances, ions can freely pass through these micropores to achieve charge exchange between the positive and negative electrodes. But when the internal temperature of the battery rises to a certain threshold, the polymer film will melt, and the micropores will close by themselves, blocking the micropores used as the transmission channel, so that the charging process is terminated, and the safety problems that may be caused by the high internal temperature of the battery are avoided. Of course the service life of the battery also ends. In this regard, there are some polymer materials to choose from. For example, polypropylene has a melting point of 155°C and polyethylene has a melting point of 130°C.
③ Positive temperature coefficient (PTC) components. This is a method that comprehensively considers the temperature and pressure inside the battery, installs PTC components in the battery, and realizes the automatic protection function of the battery. The basic principle is: during the charging process, when the temperature inside the battery rises, the PTC element will output a higher resistance, open the vent, release the gas in the battery and/or divide the battery pressure, so that the battery itself cannot continue to be charged (that is, the voltage falling between the positive and negative poles of the battery is still a safe voltage).
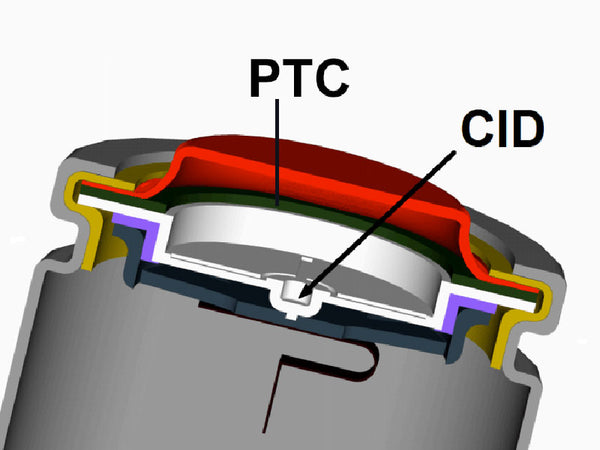
④Electronic circuit. For battery packs (even single-cell lithium-ion batteries in some special occasions), special electronic circuits are installed outside the battery to prevent overcharging and overdischarging of the battery and/or to achieve other management functions. If the temperature and/or pressure sensor of the battery is further connected with the electronic circuit that controls the charging and discharging voltage of the battery, the safety protection function of the battery can be more comprehensively realized. Of course, this will undoubtedly increase the manufacturing cost of the battery and reduce the specific energy of the battery pack.
⑤ Electrode material additives. When Li2CO3 is mixed with the positive electrode material, when overcharge occurs, the additive Li2CO3 is decomposed, releasing CO2, and increasing the internal pressure of the battery. This internal pressure activates a vent pre-placed on the top of the battery, releasing the pressure inside the battery and cutting off the charging circuit. In normal use of the battery, the additives will not significantly affect the current. Canada's Moli Energy company added 2% by mass of biphenyl to the graphite/Li2CO3 battery as an overcharge protection agent. The solid product produced by the decomposition of biphenyl is deposited on the surface of the positive electrode, which increases the internal resistance of the battery and reduces the rapid charging ability of the battery. These methods can be regarded as a combination of physical methods and chemical additive methods to prevent the battery from overcharging.
Obviously, the physical safety protection methods of the above batteries are all passive, that is, these safety protection measures usually come into play only after unsafe factors have appeared in the battery. Therefore, compared with electrolyte additives, the reliability of the above safety measures taken from battery materials, especially other than electrolytes, is relatively low.

















