As a potential cathode material, researchers have also done a lot of research on many vanadium phosphate salts. Including those with stable structure Li3V2 (PO4)3, VOPO4 and LiVPO4 and so on. Li3V2 (PO4)3 mainly exists in two forms, one is a monoclinic crystal system with stable thermodynamic properties, and the other is a rhombohedral crystal system, which can be formed by the exchange of ions in a stable sodium ion and Nasicon structure. At a voltage of about 3.80V, two lithium ions in the rhombohedral crystal system will be deintercalated, but only about 1.3 can be re-intercalated in the reverse process. The monoclinic system is currently a hotspot in the study of negative electrode materials, in which three lithium ions can be well deintercalated, and can be reversely re-inserted at a high ratio. However, the electrochemical activity of this anode material is more complicated, and a series of scan curves will appear during the charging process.
The synthesis of vanadium phosphate compound adopts solid-phase synthesis method, mixing ammonium dihydrogen phosphate, vanadium pentoxide, and lithium carbonate in a metered ratio, and heating to 300°C for 4 hours in the air to release the water vapor and ammonia gas; The product was ground again, pressed into tablets, heated at 850°C in flowing hydrogen for 8 hours, and cooled. Then heat for another 16h according to the latter process conditions to ensure complete reaction. Another method is to use CTR (carbonthermal reduction) reaction, using carbon as a reducing reagent to react stoichiometric ratios of ammonium dihydrogen phosphate, vanadium pentoxide, and lithium carbonate to produce vanadium phosphate.

Lithium vanadium phosphate
The charging potential of lithium vanadium phosphate is very high, up to 4.8V, with fast ion migration and high specific capacity, because it can reversibly extract three lithium ions from the crystal lattice. Two of the lithium deintercalation are located at 3.64V and 4.08V. The first lithium deintercalation is performed in two steps, respectively at 3.60V and 3.68V. In LixV2 (PO4)3, there is an orderly manner when x=2.5. Lithium phase. The second lithium is completed in one step. During the discharge process, a hysteresis phenomenon occurs when the first two lithiums are inserted. When the two lithiums are deintercalated, the specific charging capacity is 130mA·h/g, (theoretical value is 133mA·h/g), and the specific discharge capacity is 128mA·h/g. The platform is about V3+/V4+. The material after 4.1V deintercalation of the second lithium is more stable than deintercalation of only the first lithium. Deintercalation of the third lithium is related to V4+/V5+. The diffusion rate of lithium ions is very fast at first, and only slows down when x>2. Even under fast charging, all lithium ions can be reversibly intercalated/deintercalated. Lithium ion deintercalation is carried out step by step, and there is a phase transition for each deintercalation of a physical ion, so its charge and discharge performance is related to the potential set during charge and discharge. When the voltage is 3.0~4.8V, the charge ratio The capacity can reach 175mA·h/g, and the discharge specific capacity can reach 160mA·h/g. After 50 times of charging and discharging, the specific discharge capacity reaches 140mA·h/g. When charging and discharging at 3.0~4.5V, the specific capacity of charging for 20 weeks is 130mA·h/g. At 3.0~4.3V, the specific capacity of charging for 20 weeks is 100mA·h/g. In Li3V2(PO4)3, there are three For each lithium ion, under different voltages, the degree of delithiation is different, so the specific capacity of charging is also different. At the same time, the cycle efficiency is related to the charging voltage, and the charging efficiency is also very good at a high charging voltage. At 23℃, the actual mass specific capacity of Li3V2(PO4)3 is 10% higher than that of lithium cobalt oxide, and the theoretical volume specific capacity is lower than that of lithium cobalt oxide, which can reach 550W·h/kg, which is better than the mass ratio of lithium cobalt oxide. capacity. Even when charging and discharging at a low temperature to 10℃, the mass specific capacity of Li3V2 (PO4)3 can reach 393W·h/kg, which is higher than the mass specific capacity of lithium cobalt oxide of 315W·h/kg, so Li3V2(PO4)3 It is possible to release energy when stored at low temperatures. Through XRD analysis, after discharge, its monoclinic structure can be restored, and the reversibility is better.
In recent years, research on vanadium phosphates as cathode materials for lithium-ion batteries has gradually increased. It has been reported that both Li4P2O7 and VOPO4 can be used as cathode materials for lithium-ion batteries and have high charging potentials.
VOPO4 compound has unique and very attractive properties. Its discharge platform is about 4V, which is about 0.5V higher than LiFePO4 material. In addition, it also has higher electronic conductivity and can be used as a kind of energy more than LiFePO4. High system. It can be synthesized by removing the hydrogen atoms in VPO4·2H2O (= H2VOPO4) by heating or electrochemical stripping. VOPO4 can be obtained by placing H2VOPO4 in LiPF6/EC-DMC solution for electrochemical oxidation. VOPO4 will first be reduced to LiVOPO4 under circulating scanning current, and then Li2VOPO4 can be obtained. The specific situation is shown in Figure 1. According to the design of these reaction processes, the structures of the above four compounds are related to a certain extent. The graphic structure of building blocks can also be composed of VO6 octahedron and PO4 tetrahedron structure.
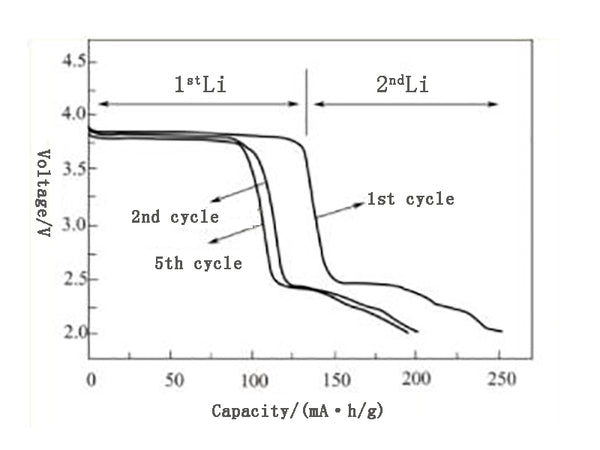
Figure 1 Electrochemical performance of ε-VOPO4 deintercalation
To make the redox potential of the material higher, you only need to heat VOPO4 in a mixture of lithium fluoride and carbon black at a temperature of 550°C for 15 minutes, and then slowly cool down at room temperature to obtain a The substance of LiVPO4F, the structure of this substance is similar to LiMPO4(OH) (M=Fe, Mn), the electrode potential is 4.2V, and the electrochemical capacity can have 0.55mol of lithium deintercalation per mole of the substance, in numerical terms, it is equal to 156mA·h/g.
LiVPO4F, the first fluorophosphate compound used as a positive electrode material for lithium-ion batteries, belongs to the triclinic system. Its structure includes a three-dimensional framework. The components are located in the phosphate tetrahedron and the oxygen-fluorine sublattice. In this structure, there are two The location of the unit cell allows lithium ions to be intercalated.

Fluorophosphate compound LiVPO4F
Using the CTR reaction, LiVPO4F can be synthesized through a two-step reaction. In the first step of the reaction, vanadium pentoxide, diammonium hydrogen phosphate, and high-surface carbon are synthesized according to the stoichiometric ratio (with 25% carbon excess) under the protection of inert gas to synthesize intermediate VPO4. The precise carbothermal reaction conditions for preparing VPO4 are determined by the semi-empirical relationship between free energy and temperature. The reduction of carbon and carbon monoxide can complete the conversion of V5+ to V3+. It can be seen from XRD that the VPO4 synthesized by this method is consistent with the spectrum obtained by the traditional synthesis method. In the CTR reaction, there is excess carbon remaining, which can maintain the stability of V3+ and is beneficial to the subsequent electrochemical process; using this method, the reaction time is short and the required temperature is lower than that of other methods. The structure is Cubic crystal system. Through elemental analysis, V and P have a definite stoichiometric ratio, which is VPO4.
In the second step of the reaction, VPO4 and lithium fluoride generate a single-phase LiVPO4F product in an argon atmosphere. At 750°C, the intermediate reacted with lithium fluoride for 15 minutes, and then rapidly cooled to produce black LiVPO4F. There was no weight loss in this reaction.
XRD analysis showed that there was no diffraction peak of lithium fluoride in the product, and it was confirmed that it had completely reacted with VPO4, and LiVPO4F was a triclinic crystal system. The particle size analysis shows that the particle size is between 10 and 40 μm. The material is a composite product, and there is excess carbon in the product. Through elemental analysis, it can be known that its determined stoichiometric formula is LiVPO4F.
When charged at 0.2C at about 23°C, its charging specific capacity is 135mA·h/g (theoretical specific capacity 156mA·h/g), which is equivalent to There are 0.87 lithium ions in the molecule of compound LiVPO4F; and there is a reversible discharge specific capacity of 115mA·h/g, which is equivalent to the discharge specific capacity of Li1-xVPO4F (x=0.74); in LiVPO4F, lithium ions are released /Intercalation is related to the V3+/V4+ redox couple, and V3+ and V4+ of vanadium are stable oxidation states, namely
LiV3+PO4F→V4+PO4F+Li+e
Under the condition of 60℃ and 0.2C, its specific capacity is above 150mA·h/g (theoretical specific capacity 156mA·h/g), indicating that its kinetic parameters are related to temperature, and all lithium can be removed to generate VPO4F compounds. Compared with other vanadium phosphate charging potentials, its charging potential is higher than 0.3V. It is precisely because it has such advantages that it is likely to replace the LiCoO2 material currently used in commercial products.