Energy is the driving force of social progress and the cornerstone of human civilization. The burning of coal has bred a profound agricultural civilization, and the development of petroleum has made a brilliant industrial civilization. With the rapid development of society and rapid population growth, traditional fossil energy sources such as coal, oil, and natural gas are gradually depleted. The energy crisis restricts the progress of the times, and new types of clean and renewable energy sources such as solar, wind, tidal energy, etc. Nuclear energy and hydroelectric power generation have become new hopes for solving the energy crisis and an important force for promoting scientific and technological progress. However, because the use of new energy is subject to intermittent constraints from the natural environment, the development of energy storage technology has become a top priority in energy use.
Chemical power source, as an efficient and clean energy conversion and storage system, including primary batteries, secondary batteries, super capacitors, flow batteries, fuel cells, etc., has attracted much attention because of its close relationship with life. Among them, lithium-ion batteries are favored because of their high specific energy, high working voltage, long cycle life, and environmental friendliness. The maturity and application of their technology have also catalyzed the leap-forward development of portable mobile devices. Due to scarcity and high production costs, traditional lithium cobalt oxide/graphite lithium-ion batteries are difficult to be used in high-power energy storage systems. Therefore, the develop a new high-quality lithium-ion battery system to further improve battery performance has become the research goal and direction of scientific researchers.
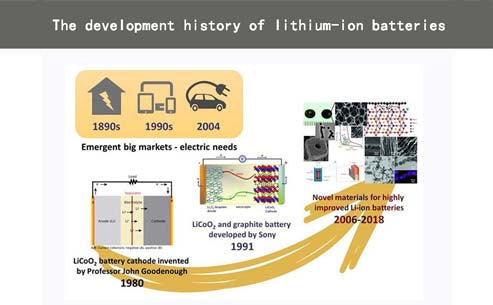
The energy crisis that appeared in the 1960s forced people to find new alternative energy sources, thereby promoting the development of lithium batteries. Lithium batteries use elementary lithium as the negative electrode, and are generally divided into lithium primary batteries (lithium primary batteries) and lithium secondary batteries (secondary lithium batteries). Li is the lightest (relative atomic mass is 6.94g/mol, and the density is 0.53g) /com³) and the most negative metal (-3.04V relative to the standard hydrogen electrode). In the 1970s, lithium batteries were used as watches, calculators and implantable medical devices with their high capacity and variable discharge rate advantages. The power supply of the device. Lithium-ion batteries are gradually developed on the basis of lithium batteries. In 1972, the concept of "electrochemical embedding" and its potential applications were reported in the conference proceedings. In the same year, Exxon in the United States pioneered a lithium-ion battery system withTS2as the positive electrode, Li metal as the negative electrode, dioxolane as the electrolyte, and lithium perchlorate as the electrolyte salt. However, the presence of Li dendrites in the system may cause explosions. Hidden dangers. Until 1991, the Japanese company Sony based on the early discovery of highly reversible low-voltage Li insertion/extraction carbon materials, and the LixMO2 (where M=Co, Ni, etc.) layered lithium-rich compound discovered by Goodenough et al., using LiCoO2 as the The positive electrode and the C material as the negative electrode have developed a LiCoO2/C battery system. Li exists in the ionic state rather than the metallic state in this system, which inhibits the generation of Li dendrites to a certain extent. In theory, the new system is better than lithium metal batteries. safer. The battery voltage is higher than 3.6v, which is three times that of alkaline batteries, and the energy density is as high as 120-150w·h/kg, which is 2-3 times that of nickel-cadmium batteries, so it shines in high-performance portable electronic equipment. In 1999, polymer electrolyte was introduced into the lithium-ion battery system, and thin-film battery technology was developed. The polymer lithium-ion electrolytic cell was called a plastic lithium-ion (PLiON) battery and entered the market. Since then, various lithium-ion batteries with different systems have emerged in response to market demand. In the past 30 years, lithium-ion batteries have led the market in sales of portable and portable electronic device technology.
Chemical power source, as an efficient and clean energy conversion and storage system, including primary batteries, secondary batteries, super capacitors, flow batteries, fuel cells, etc., has attracted much attention because of its close relationship with life. Among them, lithium-ion batteries are favored because of their high specific energy, high working voltage, long cycle life, and environmental friendliness. The maturity and application of their technology have also catalyzed the leap-forward development of portable mobile devices. Due to scarcity and high production costs, traditional lithium cobalt oxide/graphite lithium-ion batteries are difficult to be used in high-power energy storage systems. Therefore, the develop a new high-quality lithium-ion battery system to further improve battery performance has become the research goal and direction of scientific researchers.
The energy crisis that appeared in the 1960s forced people to find new alternative energy sources, thereby promoting the development of lithium batteries. Lithium batteries use elementary lithium as the negative electrode, and are generally divided into lithium primary batteries (lithium primary batteries) and lithium secondary batteries (secondary lithium batteries). Li is the lightest (relative atomic mass is 6.94g/mol, and the density is 0.53g) /com³) and the most negative metal (-3.04V relative to the standard hydrogen electrode). In the 1970s, lithium batteries were used as watches, calculators and implantable medical devices with their high capacity and variable discharge rate advantages. The power supply of the device. Lithium-ion batteries are gradually developed on the basis of lithium batteries. In 1972, the concept of "electrochemical embedding" and its potential applications were reported in the conference proceedings. In the same year, Exxon in the United States pioneered a lithium-ion battery system withTS2as the positive electrode, Li metal as the negative electrode, dioxolane as the electrolyte, and lithium perchlorate as the electrolyte salt. However, the presence of Li dendrites in the system may cause explosions. Hidden dangers. Until 1991, the Japanese company Sony based on the early discovery of highly reversible low-voltage Li insertion/extraction carbon materials, and the LixMO2 (where M=Co, Ni, etc.) layered lithium-rich compound discovered by Goodenough et al., using LiCoO2 as the The positive electrode and the C material as the negative electrode have developed a LiCoO2/C battery system. Li exists in the ionic state rather than the metallic state in this system, which inhibits the generation of Li dendrites to a certain extent. In theory, the new system is better than lithium metal batteries. safer. The battery voltage is higher than 3.6v, which is three times that of alkaline batteries, and the energy density is as high as 120-150w·h/kg, which is 2-3 times that of nickel-cadmium batteries, so it shines in high-performance portable electronic equipment. In 1999, polymer electrolyte was introduced into the lithium-ion battery system, and thin-film battery technology was developed. The polymer lithium-ion electrolytic cell was called a plastic lithium-ion (PLiON) battery and entered the market. Since then, various lithium-ion batteries with different systems have emerged in response to market demand. In the past 30 years, lithium-ion batteries have led the market in sales of portable and portable electronic device technology.