
The manganese source, lithium source (LiOH·H2O) and mineralizer are sealed in a high pressure reactor according to a certain ratio, and the synthesis reaction temperature is controlled not to be higher than 220 ℃ by external heating to synthesize orthogonal layered lithium manganese oxide.
Nanoparticles prepared by solvothermal method usually have the characteristics of uniform phase, high purity, good crystal form, monodispersion, and controllable shape and size. In the process of solvent heat treatment, temperature, pressure, treatment time, the type of precursor used and the pH value of the system have a great influence on the particle size and morphology of the powder, as well as the reaction rate and the crystal form of the product. Using γ-MnOOH, Mn2O3, Mn3O4, and MnO2 as raw materials, o-LiMnO2 can be effectively synthesized through a hydrothermal reaction process. The consideration of low-temperature and high-pressure conditions using solvothermal allows Li+ to effectively migrate into the manganese compound to form lithium manganese. Oxygen compounds.
Now take the solvothermal synthesis of lithium manganese oxide compounds as an example. Synthesis of Mn3O4-δ: Put a certain amount of MnO2 powder and absolute ethanol in the reactor and seal it, heat it for 12 hours at a certain temperature, and then cool it down to room temperature naturally with the furnace to obtain a reddish brown product.
Synthesis of LixMnO2: Put a certain amount of MnO2 powder, LiOH·H2O and NaOH into a 100mL stainless steel reactor lined with polytetrafluoroethylene, and add solvent absolute ethanol to 75% of the container volume. After the reaction kettle was sealed at a certain temperature and heated for 12 hours, it was naturally cooled to room temperature along with the furnace. The gray-brown precipitate was collected, washed with absolute ethanol, and the product was dried in a vacuum drying oven at 80°C for 24 hours.
High-purity tetragonal Mn3O4-δ nanocrystals are suitable for making soft magnetic materials, such as high-frequency converters, magnetic heads, and manganese-zinc ferrite cores. Mn3O4-δ is also suitable for preparing lithium-ion battery cathode materials spinel lithium manganese The raw material of oxygen solid solution. The non-stoichiometric Mn3O4-δ is composed of an octahedral Mn3O4-δ phase and a tetrahedral MnO phase. The oxygen vacancies in the structure are its catalytic active center. Since Mn3O4-δ is an intermediate compound for the preparation of lithium manganese oxide electrode materials, clarify the existence form, bonding mode and reaction mechanism of various species of manganese oxide in the preparation process, and study the phase transition process and reaction of preparing high-purity Mn3O4 The history and grain growth kinetics are very necessary. According to the principle, the preparation method of Mn3O4 can be divided into two types: oxidation of low-valence manganese and reduction of high-valence manganese. Generally, calcination of various manganese oxides, hydroxides, nitrates, carbonates and sulfates at a high temperature of 1000 ℃ can obtain Mn3O4, and only large particles of Mn3O4 can be obtained by the calcination method. Liquid phase method or hydrothermal method can be used to synthesize Mn3O4 with uniform particle size and controllable morphology.
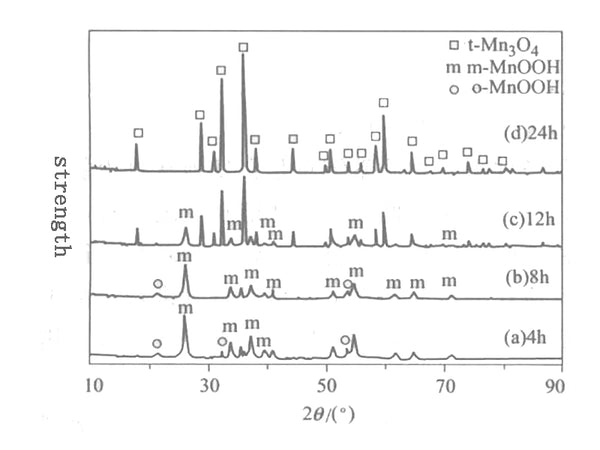
Figure 1 XRD pattern of the product when MnO2 reacts with ethanol
Figure 1 shows the XRD pattern of the product obtained after 0.2mol MnO2 reacted with ethanol at 160°C for different times. Figure 1 shows that MnO2 can be completely converted into monoclinic and orthorhombic MnOOH after 4 hours of reaction at 160℃; after 8 hours of reaction, the product is dominated by monoclinic MnOOH phase, and there is still a small amount of positive Cross MnOOH exists; after reacting for 12h at 160℃, the orthogonal o-MnOOH disappears, and the amount of monoclinic m-MnOOH phase is also significantly reduced. The new phase produced is the tetragonal phase t-Mn3O4; after reaction for 24h, m-MnOOH is All phases disappeared, and a tetragonal phase t-Mn3O4 with pure phase and complete crystal shape was obtained. From the above analysis, it can be seen that the synthesis reaction time at 160℃ has a great influence on the phase of the solvothermal product. Under the conditions, the solvothermal synthesis reaction time is prolonged, and the phase changes as follows: MnO2→m-MnOOH+o-MnOOH→m-MnOOH+t-Mn3O4→t-Mn3O4. Understand this phase change process of solvothermal synthesis of Mn3O4. The use of solvothermal to synthesize lithium manganese oxide electrical materials has a practical guiding role.
From the experimental phenomenon, the color of the solid phase changed from black MnO2 to brown MnOOH after 4h solvothermal reaction at 160℃; after 24h solvothermal reaction, the color of the solid phase changed from black MnO2 to reddish brown Mn3O4, Its XRD diffraction peak corresponds exactly to the JCPDS card (24-0734) of t-Mn3O4, and there is no impurity peak.
When the synthesis reaction time is short, the weak reducing agent C2H5OH is oxidized to CH3CHO, and MnO2 is reduced to MnOOH. The chemical reaction formula is as follows:
2MnO2+C2H5OH→2MnOOH+CH3CHO
When the synthesis reaction time is long, MnOOH dehydrates or continues to react with ethanol to produce Mn2O3 or Mn3O4. The possible chemical reaction formula is:
2MnOOH→Mn2O3+H2O
6MnOOH→Mn3O4+3H2O+(1/2)O2
or:
2MnOOH+C2H5OH→Mn2O3+CH3CHO+H2O
6MnOOH+C2H5OH→2Mn3O4+CH3CHO+4H2O
Therefore, the general chemical reaction formula is:
6MnO2+3C2H5OH→2Mn3O4+3CH3CHO+(1/2)O2+3H2O
2MnO2+C2H5OH→Mn2O3+CH3CHO+H2O
or:
3MnO2+2C2H5OH→Mn3O4+2CH3CHO+2H2O
2MnO2+2C2H5OH→Mn2O3+2CH3CHO+H2O
From the second law of thermodynamics, we know that under isothermal and pressure conditions, the material system always spontaneously transitions from a state with a higher free energy to a state with a lower free energy. That is, only the process of reducing the free energy can proceed spontaneously, or only when the free energy of the new phase is lower than the free energy of the old phase, the old phase can spontaneously transform into the new phase. The above-mentioned reactions are all less than zero by thermodynamic calculation, that is, these four reactions can all proceed spontaneously under certain conditions. Because Mn3O4 can exist more stably than Mn2O3, the final inorganic product is Mn3O4 nanocrystal. This thermodynamic analysis result is consistent with the XRD characterization result.
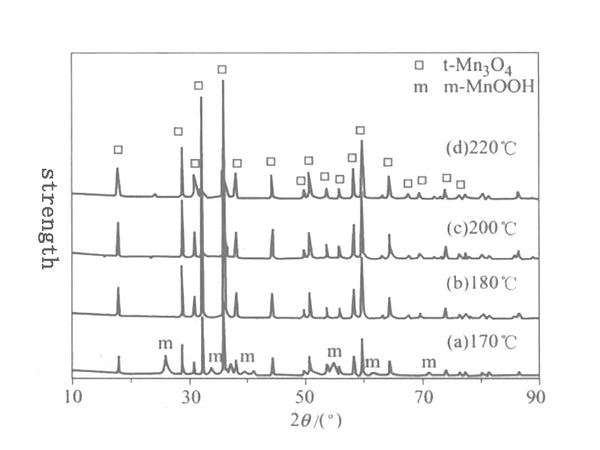
Figure 2 XRD patterns of solvothermal reaction 24h Mn3O4 samples at different temperatures
Figure 2 shows the XRD pattern of the solvothermal reaction at different temperatures for 24h. After solvothermal reaction at 180~220℃ for 24h, the crystal form of the product is all tetragonal Mn3O4. Use the least square method to calculate the unit cell parameters of the tetragonal system Mn2O3 according to the following formula, and calculate the grain size (D) from the XRD diffractometer's self-carrying degree and the XRD half-width of the XRD. The structure parameters of the synthesized product at a temperature of 180~220℃ See Table 1, where c/a=1.63, indicating that the synthesized Mn3O4 crystal has tetragonal symmetry.
1/(dhkl²)=[(h²+k²)/a²]+(l²/c²)
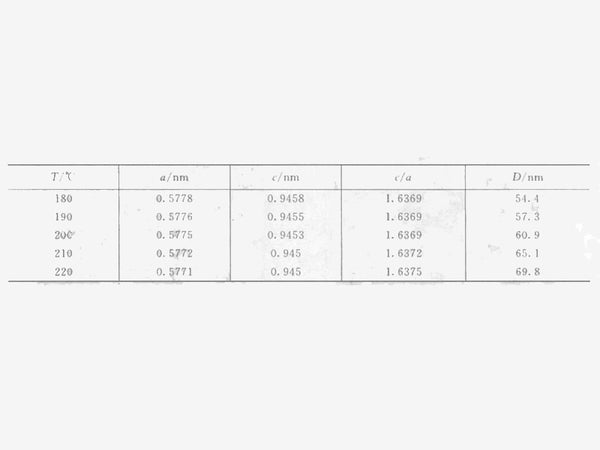
Table 1 Unit cell parameters and grain size of Mn3O4 prepared by solvothermal reduction method at different temperatures
The SEM images of Mn3O4 particles generated under 180℃ and 200℃ solvothermal conditions show that the morphologies of the two samples are all tetragonal three-dimensional shapes, and the Mn3O4 particles are in agglomerated state. The particle size displayed by the SEM is higher than the grain size calculated by the XRD data. About ten times larger, the particle size of the sample synthesized at 180°C is significantly smaller than that of the sample synthesized at 200°C.
Under airtight conditions, the equilibrium vapor pressure of a liquid is determined by the temperature, and generally has nothing to do with the amount of liquid and the size of the container. According to the relationship between the heat of vaporization and the boiling point:
LnP=[﹣(△vap·THm)/RT]+C
The corresponding relationship between boiling point and saturated vapor pressure can be obtained. In the formula, P is the saturated vapor pressure; T is the thermodynamic temperature; R is the gas constant; C is the constant; Δvap.THm is the heat of vaporization at a certain temperature.
It can be seen from the above formula that the vapor pressure of a liquid generally has the characteristic of a sharp rise with temperature. Due to the large excess of ethanol added, the partial pressure of ethanol in the reactor increases with the increase of the reaction temperature. It can be speculated that the growth rate of crystal grains will increase with the increase of the reaction temperature. It can be seen from Table 1 that the grain size calculated according to the XRD half-width is increased from 54.4nm at 180°C to 69.8nm at 220°C.
In the research on the solvothermal synthesis of t-Mn3O4, it was found that with the increase of the solvothermal synthesis temperature and the prolongation of the reaction time, the phase transition occurred as follows: MnO2→m-MnOOH +o-MnOOH→m-MnOOH+t-Mn3O4 →t-Mn3O4, in which o-MnOOH is unstable and reacts first, so the orthogonal layered lithium manganese oxide can be obtained by adding LiOH.
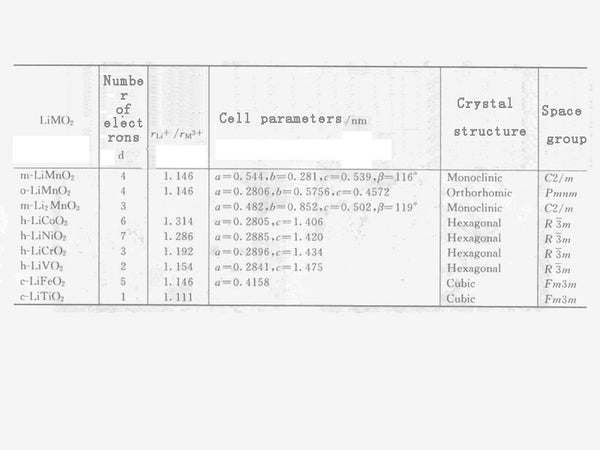
Table 2 The relationship between LiMO2 cation radius ratio and unit cell parameters, crystal structure, and space group.
The relationship between the cation radius ratio of the layered lithium manganese oxide and other LiMO2 solid solutions and the crystal structure and unit cell parameters are shown in Table 2. It can be seen from Table 2 that according to the ratio of cation radius, LiMnO2 rock salt can be roughly divided into three types: monoclinic system, orthorhombic system and hexagonal system with different crystal symmetry. If the ratio of rLi+/rM3+ is large, the layered rock salt stable phases LiCoO2, LiNiO2 and LiCrO2 of the hexagonal crystal system can be obtained.

Figure 4 SEM image of LiCoPO4 prepared by hydrothermal method
(Reaction conditions: 220°C for 5 hours, pH=8.50)
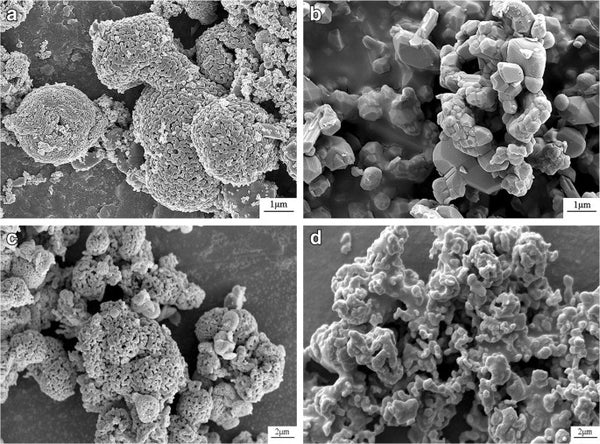
Figure 5 SEM image of LiMn2O4 prepared by hydrothermal method
Figures 3 and 4 are SEM images of LiCoPO4 and LiMn2O4 prepared by hydrothermal method, respectively.
















