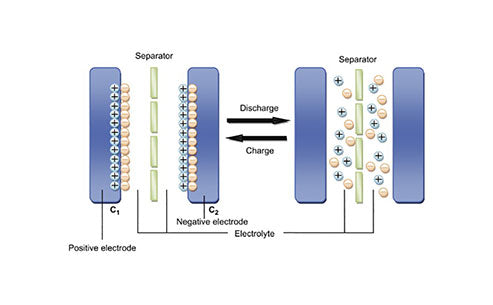
As a lithium-ion battery electrolyte solvent, propylene carbonate (PC) has the advantages of low price, low melting point (-49.2°C), high dielectric constant, high chemical stability, high flash point and boiling point, and wide electrochemical window. The PC-based electrolyte has both good low-temperature and high-temperature performance, which helps to improve the low-temperature performance and safety performance of lithium-ion batteries. However, when the PC-based electrolyte is used in combination with graphite anode materials that are widely used in commercial lithium-ion batteries with the advantages of low cost, high capacity, and flat lithium insertion platform, PC molecules will be embedded in the graphite layer together with solvated lithium ions, and undergo a violent reduction and decomposition reaction in it, as shown in Figure 1, causing the peeling of the graphite flakes and destroying the graphite electrode structure. An effective way to solve this problem is to add film-forming additives to the electrolyte, which takes precedence over the decomposition of PC (or easier to decompose than PC), and forms an effective, dense and stable SEI film on the surface of the graphite anode material to prevent the co-intercalation of PC and solvated lithium ions. Therefore, the basic principle of using additives to improve the cycle stability of PC-based electrolytes is that the additives must decompose above the potential at which lithium ions are inserted into the graphite electrode, and form a uniform, dense and stable SEI film on the graphite surface. In this way, when the discharge voltage drops to the lithium-intercalation potential, the layered peeling, powdering, and shedding of graphite carbon caused by the co-intercalation of PC and the reduction of PC can be avoided.

Figure 1 - Schematic diagram of the intercalation of the solvent together with the solvated lithium ions into the graphite layers
Vinyl butyrate (VB), vinyl hexanoate, vinyl benzoate, vinyl crotonate and vinyl pivalate (vinyl pivalate) can be used as additives for PC and liquid electrolyte systems. The addition of vinyl butyrate to the PC-based electrolyte can significantly inhibit the decomposition of the electrolyte on the graphite electrode and improve the electrochemical performance of the battery. Calculations show that the relationship between the lowest unoccupied molecular orbital (LUMO) energy of VB and the reduction potential of the additive is basically linear. To test the role of vinyl acetate, divinyl adipate and allyl methyl carbonate as an additive for PC-based electrolytes in three kinds of graphite carbon (natural graphite, MCMB 6-28 and MCF) commonly used in lithium-ion batteries, and to evaluate the feasibility of direct two-electron reduction of these solvents, and evaluate the feasibility of direct two-electron reduction of these solvents, it is not found that there is an energy barrier when Li2CO3 and 1,4-butadiene (1,4-butadiene) are generated by the decomposition of VEC. On the contrary, the decomposition of EC or PC needs to cross the 0.5eV energy barrier. The reductive decomposition of VEC is prone to produce Li2CO3, which may explain why VEC can be used as a passivation agent for lithium-ion batteries.
Using the same volume of fluoroethylene carbonate (fluoro-EC) and co-solvent electrolyte, the capacity loss caused by the decomposition of the electrolyte during the first lithium insertion process of graphite is reduced to 85mA·h/g. In a ternary solvent system containing equal volumes of EC and PC, the volume fraction of fluoroethylene carbonate can be further reduced to 0.05. Lithium-ion batteries using electrolytes containing fluoroethylene carbonate, PC and EC solvents have a long cycle life. After 200 cycles, the battery capacity is reduced to 37% of the original, and the current efficiency of the battery is 100%. Therefore, the problem of low current efficiency of the battery when using vinyl chloride carbonate (chloro-EC) instead of vinyl fluoride carbonate is solved.
The wet chemical method is used to deposit nano-silver particles on graphitized mesophase carbon microbeads (MCMB) with a low coverage rate, and it was found that the deposition of nano-silver particles promoted the formation of a stable SEI film on a carbon electrode using a PC-based electrolyte. As a result, the intercalation and deintercalation of lithium ions become reversible.
Vinylene carbonate (VC) can be used as a highly reactive film-forming additive to minimize the decomposition of the electrolyte and improve the charge and discharge efficiency and cycle characteristics of potassium ion batteries. But VC is an extremely unstable compound. The molecular structure of vinyl ethylene carbonate (VEC) is very similar to that of VC, but because the double bond in VEC is directly connected to the power supply group CH outside the ring, the electron cloud density on the double bond increases, which is conducive to stabilizing the double bond. The double bond in VC is directly connected to the electron withdrawing group OCOO in the ring, which reduces the electron cloud density on the double bond, which causes the double bond to be extremely unstable and prone to polymerization.
When used as a solvent, VEC has a high dielectric constant, and has a high boiling point and flash point, which is conducive to improving the safety of lithium-ion batteries. The physical and electrochemical properties, reduction decomposition mechanism and electrochemical behavior of VEC as a film-forming additive of PC-based electrolytes and electrochemical behaviors under different graphite morphologies and charge and discharge current densities have been carried out in detailed experiments and theoretical studies, and it is found that VEC begins to decompose at 1.35V, and its product can form a dense and stable SEI film on the sheet graphite, which can effectively prevent the co-intercalation of PC and solvated lithium ions between the graphite layers. VEC has a high oxidation potential, and has good compatibility with the cathode material LiMn2O4. FTIR, XPS and GC-MS research results show that the main reduction products of VEC are lithium alkoxide ROLi (R is alkyl), alkyl ester lithium ROCO2Li, lithium carbonate, lithium carbon compounds and 1,3-butadiene, etc. . According to theoretical calculations, the reductive decomposition products of VEC mainly include lithium carbonate, LiOCH(CH-CH2)CH2OCOCH(CH=CH2)CH2OCO2Li, LiO2COCH2CH(CH=CH2)OCO2Li, LiO2COCH2CH(CH=CH2) and LiCH(CH=CH2)CH2OCO2Li and so on.
The electrochemical behavior of graphite electrodes in PC/VEC electrolyte highly depends on the morphology and charge-discharge current density of graphite. At low current density, the reduction and decomposition products of VEC can form an effective, sufficient and stable SEI film on the surface of the sheet graphite but cannot form an effective and sufficient SEI film on the surface of the spherical graphite MCMB. Only at high current density, the reduction and decomposition products of VEC can form an effective and sufficient SEI film on the surface of flake graphite and spherical graphite MCMB. By simply controlling the charge and discharge current density, the co-intercalation of PC and solvated lithium ions into the spherical MCMB can be significantly inhibited. The main product of reductive decomposition of VEC at low current density is long-chain alkyl ester lithium LiOROCO2Li, while the main product of reductive decomposition at high current density is inorganic Li2CO3. It is this specific reduction and decomposition product that determines the different electrochemical behaviors of graphite materials with different morphologies.
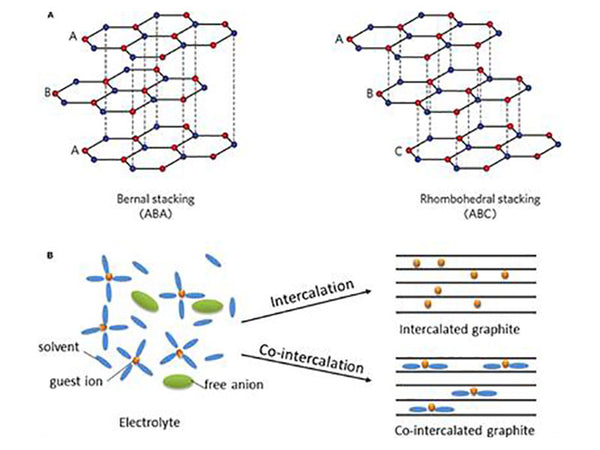
Co-intercalation involves the insertion of solvated ions
(A) Schematic illustration of graphite structure; (B) Schematic diagrams of the coordination structure in electrolyte, the intercalated graphite and the co-intercalated graphite, respectively.
Although PC has many advantages and adding a small amount of suitable film-forming additives can solve its compatibility with graphite anode materials, there are still many problems that need to be solved urgently if it is applied in actual lithium-ion batteries. The first is that the viscosity of PC is too high, which directly causes the conductivity of the electrolyte system [(2~6)×10-3S/cm] to be lower than that of the current commonly used electrolyte system [(10~18)×10-3S/cm]. Although the conductivity of the PC-based electrolyte can meet the requirements of ordinary lithium-ion batteries, its conductivity is still lower if it is used in power-type lithium-ion batteries; in addition, the penetration of the PC-based electrolyte with the electrode material is very poor, so the utilization rate of the active material is not high. Although some low-boiling chain carbonates, such as DMC, DEC, EMC, etc., can be added to the PC-based electrolyte to improve the low-temperature performance of the system, two ideas that may help solve this problem are proposed: one is to add a small amount of suitable surfactants to the PC-based electrolyte to reduce the surface tension of the electrolyte and increase the degree of infiltration of the electrolyte and the electrode surface; the second is to prepare the PC-based electrolyte into a system similar to "beer", add some gas to the PC-based electrolyte, and require the pressure to be maintained within the acceptable range of the battery. The gas is best to choose a neutral gas, such as CO2 (PC can absorb a large amount of CO2, generally use PC to purify CO2). The electrolyte system may have the following advantages: ①Adding a very small amount of CO2 to the PC-based electrolyte can significantly improve the SEI film on the surface of the graphite anode material, preventing the co-intercalation of PC and solvated lithium ions between the graphite layers; ②It can improve the infiltration state of the electrolyte and the electrode surface; ③It can inhibit side reactions that produce gas; ④It can improve the safety performance of lithium-ion batteries; ⑤It may have higher conductivity, etc.