Polymer materials have always been used as insulating materials in the past, but since Mac Diarmid discovered conductive polyacetylene, it has created a new situation of polymer materials as conductive materials. Because some conductive polymer materials have reversible electrochemical activity and their specific capacity is comparable to that of metal oxide electrodes, they are playing a very important role in chemical power sources and have broad application prospects. At present, there are many researches on the application of lithium-ion batteries, and products have come out in various countries.
Conductive polymer molecules are composed of many small, recurring structural units. When a certain voltage is applied to both ends of the conductive polymer molecule, current flows through the material. The structural feature of the conductive polymer molecule is that there is a large linear conjugated π-electron system in the molecule, which can provide conditions for the delocalization of carrier free electrons. When the electric capacity is constant, compared with inorganic materials, the conductive polymer as the battery electrode material can make the battery have the advantages of light weight, non-corrosion, and repeated charging and discharging.
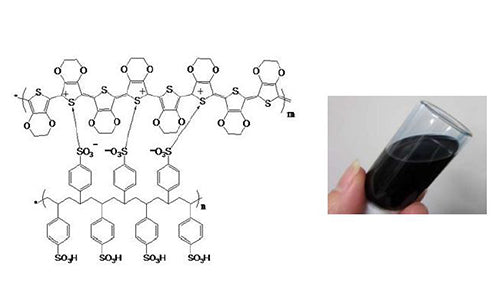
Compared with other cathode materials, polymer cathode materials have the following advantages: good processability, can be processed into suitable shapes according to needs, and can also be made into membrane batteries; weight ratio energy is large; unlike metal electrodes, it is not easy to produce dendrites. Internal short circuit: Generally, the inorganic electrode only produces reduction reaction on the electrode surface, while the electrode made of polymer has the electrode reaction inside the entire porous polymer matrix, so the specific surface area of the electrode is large and the specific power is large.

Polymer cathode material
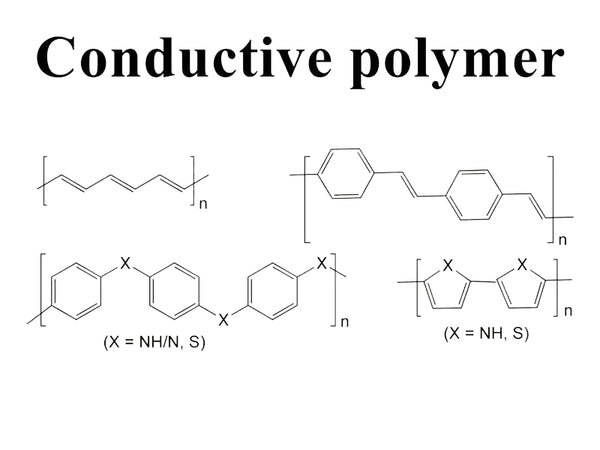
Currently, the most researched polymer cathode materials include polyaniline (PAn), polypyrrole (PPY), polyacetylene (PA) and polyparaphenylene (PPP). PAn is considered to be the most promising cathode material for rechargeable batteries for its excellent electrochemical performance, good redox reversibility, good environmental stability, and easy electropolymerization or chemical oxidation synthesis. As a new type of organic electronic materials, it is currently becoming a hot spot in research and development strategies at home and abroad. In 1987, Japan put button Li-Al/LiBF4-PC/PAn batteries on the market, becoming the first commercial plastic battery.
PAn is a conductive material. To make electrons move freely in the conjugated π-electron system, the first step is to overcome the energy level difference between the full band and the empty band that determines the conductivity of the conductive polymer. The doping can change the electron occupancy in the energy band. situation. During the doping process of PAn, the dopant is inserted between the molecular chains, and the electron occupancy of the molecular orbital is changed through the transfer of electrons, and the energy band structure itself is also changed. The electrolyte of the polymer lithium ion battery gradually enters the PAn molecule and supplies a sufficient amount of anions to increase its doping amount. Taking Li/PEO-LiClO4/PAn battery as an example, the electrode reaction is as follows:
The positive reaction is: PAn+ClO4﹣charge↔discharge PAn+ClO4﹣+e
The negative reaction is: Li++e charge↔discharge Li
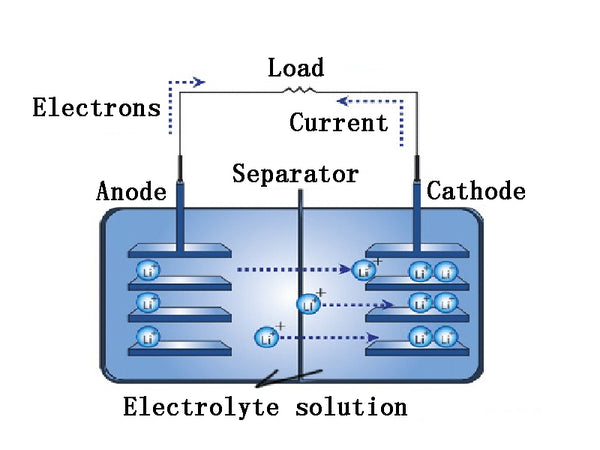
When the battery is discharged, the lithium atoms of the negative electrode lose electrons to form lithium ions. The lost electrons are attracted by the positive electrode's electric field and enter the positive electrode through the external circuit. The negative electrode generates lithium ions by diffusion and enters the electrolyte. The ClO4- in the positive electrode PAn enters the electrolyte. When the battery is charged, ClO4-doped into PAn, the lithium ions in the electrolyte are reduced to metallic lithium, which is deposited on the lithium negative electrode, and electrons flow from the positive electrode to the negative electrode through the external circuit.
PAn can be prepared by electrochemical polymerization and chemical oxidation methods. Electrochemical polymerization is a method of preparing high polymer materials developed in recent years. It uses the electrode potential as the initiation and reaction driving force of the polymerization reaction to carry out the polymerization reaction on the surface of the electrode and directly generate the polymer. The polymerization of aniline in water-soluble electrolyte can be carried out by electrochemical methods such as potentiostatic method, galvanostatic method, potentiostatic scanning method, pulse polarization method, etc. The auxiliary electrode can be platinum electrode, nickel lead electrode, iron electrode, etc.
The electrolyte solution can be H2SO4, HClO4, HBF4, HCl, etc. PAn is electrodeposited on the substrate material, such as Au, Pt, stainless steel, SnO2 sheet and carbon electrode. The factors that affect the electrochemical polymerization of aniline include the pH value of the solution, the type and concentration of anions in the electrolytic solution, the concentration of aniline monomer, and the surface state of the electrode. Chemical oxidation is a method of oxidizing and polymerizing aniline with an oxidizing agent under certain conditions to form a polymer. The synthesized PAn is protonated in an acidic solution, and part of it is in an oxidized state. This protonated compound is neutralized with a base and converted into a corresponding salt. The more commonly used oxidants are persulfate, hydrogen peroxide, chlorate, dichromate, hypochlorite and so on. Factors affecting chemical polymerization include solvent system and acidity, anion species, aniline concentration, oxidant species, concentration and reaction temperature. The mixing of PAn powder prepared by chemical oxidation with carbon black and polytetrafluoroethylene can improve the electrical conductivity of the material and increase its mechanical stability.
electrolyte solution
The properties of PAn in terms of specific capacity, specific energy, electrode potential, coulombic efficiency, cycle characteristics, chemical stability, etc. indicate that it can be used as an electrode material for high-energy battery research and development. However, as a new type of organic electronic material, the research of PAn is still in the exploratory stage of the laboratory. The understanding of its electronic behavior as an electrochemically active material is still limited, and its structure, aggregation form, conductivity and mechanism are related. The research still needs to be in-depth.
Polyheterocyclic conductive polymers such as polypyrrole and polythiophene can also be used as the positive electrode of lithium ion batteries. These materials can be easily obtained by electrochemical polymerization. Lithium-ion batteries using these polymers as electrodes have the advantages of good cycle characteristics, high charge and discharge efficiency, and high coulombic efficiency. However, these polymer electrodes have serious self-discharge, resulting in continuous power loss.