
What is the cathode material in lithium ion batteries
The electrode is the core of the battery and consists of an active material and a conductive skeleton. The positive and negative active materials are the source of electricity generation and an important part of determining the basic characteristics of the battery. For example, in lithium-ion batteries, the current commercial lithium-ion battery cathode active material is generally LiCoO2, the current hot spot of scientific research is working towards cobalt-free materials, and some cathode materials on the market also use LiMn2O4, etc.
The non-electrostatic force inside the power supply is the work done in the process of moving a unit of positive charge from the negative pole of the power supply to the positive pole through the internal circuit. The symbol of electromotive force is ℇ, and the unit is volts (V). The power source is a device that converts other forms of energy into electrical energy. To maintain a constant current in the circuit, only the electrostatic field force is not enough, but also non-electrostatic force. The power supply provides non-electrostatic force, which moves the positive charge from a low electric potential to a high electric potential. The process by which the non-electrostatic force pushes the charge to do work is the process by which other forms of energy can be converted into electrical energy.
Electromotive force is a physical quantity that characterizes the electrical energy produced by a power source. Different power sources have different sources of non-electrostatic force and different forms of energy conversion. For example, the non-electrostatic force of the chemical electromotive force (dry battery, button battery, storage battery, lithium ion battery, etc.) is a chemical action, and the size of the electromotive force has nothing to do with the size of the power source; the non-electrostatic force of the generator is the force of the magnetic field on the moving charge; The non-electrostatic force of the photoelectromotive force (photocell) comes from the internal photoelectric effect; and the piezoelectric electromotive force (crystal piezoelectric ignition, crystal microphone, etc.) comes from the polarization phenomenon caused by mechanical work.
When the external circuit of the power supply is disconnected, the non-electrostatic force inside the power supply is balanced with the electrostatic field force, and the voltage across the positive and negative poles of the power supply is equal to the electromotive force of the power supply. When the external circuit is turned on, the terminal voltage is less than the electromotive force.
For lithium-ion batteries, the requirements for active materials are: First, the electromotive force of the battery is high, that is, the more positive the standard electrode potential of the positive electrode active material, the more negative the standard electrode potential of the negative electrode active material, and the higher the electromotive force of the battery. .
Take the lithium ion battery as an example. It usually uses LiCoO2 as the positive electrode active material and carbon as the negative electrode active material, which can obtain an electromotive force as high as 3.6V or more; secondly, the active material has a spontaneous reaction ability, the better; the weight ratio of the battery The capacity and volume ratio are larger. The theoretical weight ratio capacities of LiCoO2 and graphite are both larger, 279mA·h/g and 372mA·h/g respectively; moreover, the active material is required to have high stability in the electrolyte, so that Reduce the self-discharge of the battery during storage, thereby improving the storage performance of the battery; in addition, the active material requires higher electronic conductivity to reduce its internal resistance; of course, from the economic and environmental considerations, the active material is required to have a wide range of sources , Cheap and friendly to the environment.
In addition to the above-mentioned requirements, the selection of positive electrode active materials for lithium-ion batteries has its special requirements. Specifically, the selection of positive electrode materials for lithium-ion batteries must follow the following principles.
①The positive electrode material has a large Gibbs white energy, so as to maintain a large potential difference with the negative electrode material to provide battery operating voltage (high specific power).
② The Gibbs free energy change during the lithium ion intercalation reaction ∆G is small, that is, the amount of lithium ion intercalation is large and the dependence of the electrode potential on the amount of intercalation is small, which ensures the stable operating voltage of the lithium ion battery.
③Wide range of insertion/deintercalation of lithium ions and a considerable amount of intercalation/deintercalation of lithium ions (large specific capacity).
④The positive electrode material needs to have a large pore "tunnel" structure to facilitate the insertion/escape of lithium ions in the charge and discharge process.
⑤Lithium ion has a large diffusion coefficient and mobility coefficient in the "tunnel" to ensure a large diffusion rate and good electronic conductivity to increase the maximum operating current of the lithium ion battery.
⑥The positive electrode material has a large interface structure and a more apparent structure to increase the space position of lithium insertion during discharge and increase its lithium insertion capacity.
⑦The chemical and physical properties of the positive electrode material are uniform, and its abnormality is very small to ensure the good reversibility of the battery (long cycle life).
⑧No chemical or physical reaction with electrolyte.
⑨ It has good compatibility with electrolyte and high thermal stability to ensure the working safety of the battery.
⑩It has light weight and is easy to make suitable electrode structure in order to improve the cost performance of lithium ion battery.
⑪Non-toxic, inexpensive, and easy to prepare.
In addition to LiCoO2, the commonly used positive electrode active materials also include LiMn2O4.
LiCoO2, a commercial cathode material for lithium-ion batteries, belongs to the α-NaFeO2 structure. The synthesis methods of layered rock salt lithium cobalt oxide mainly include high temperature solid phase method, low temperature co-precipitation method and gel method. The more mature method is high temperature solid phase method.
Cobalt is a strategic element. The global reserves are very limited. It is expensive and toxic. Therefore, the cost of lithium-ion batteries using LiCoO2 as the positive electrode active material is relatively high; in addition, in LiCoO2, the amount of reversibly deintercalating lithium is 0.5 ~0.6mol, when the lithium released during overcharge is greater than 0.6mol, the excess lithium is deposited on the negative electrode in the form of elemental lithium, which will also bring safety hazards. The CoO2 produced after LiCoO2 overcharge has a strong catalytic activity for electrolyte oxidation. At the same time, the initial decomposition temperature of CoO2 is low (about 240℃) and the heat released is large (1000/g), so LiCoO2 is used as the cathode material for lithium ion batteries There are also serious safety hazards, and it is only suitable for small-capacity single batteries to be used alone.
Compared with metallic cobalt, metallic nickel is much cheaper. The world has proved that the recoverable reserves of nickel are about 14.5 times that of cobalt, and its toxicity is also lower. Due to the close chemical properties of Ni and Co, LiNiO2 and LiCoO2 have the same structure. Both compounds belong to the α-NaFeO2 two-dimensional layered structure, which is suitable for the extraction and insertion of lithium ions. LiNiO2 does not have the limitations of overcharge and overdischarge, and has good high temperature stability, low self-discharge rate, low pollution, and low requirements for electrolyte. It is a promising cathode material for lithium-ion batteries; however, LiNiO2 During the charging and discharging process, its structure is not stable, and the manufacturing process conditions are harsh, and it is not easy to prepare LiNiO2 with a stable α-NaFeO2 two-dimensional layered structure.
LiNiO2 is usually synthesized by high-temperature solid-phase reaction, using LiOH, LiNO3, Li2O, Li2CO3 and other lithium salts and Ni(OH)2, NiNO3, NiO and other nickel salts as raw materials. The molar ratio of Ni to Li is (1:1.1)~ (1:1.5), after the reactants are mixed uniformly, they are pressed into tablets or pellets and calcined in an oxygen-rich atmosphere at 650-850°C for 5-16 hours.
Compared with lithium cobalt oxide and lithium nickel oxide, lithium manganese oxide has good safety and good overcharge resistance. The raw material manganese is one of the most promising cathode materials due to its abundant resources, low price and non-toxicity. There are two main types of lithium manganese oxides, namely LiMnO2 with a layered structure and LiMn2O4 with a spinel structure.
The spinel LiMn2O4 belongs to the cubic crystal system and has the Fd3m space group; its theoretical capacity is 148mA·h/g, in which oxygen atoms form a face-centered cubic close packing (ccp), and lithium and manganese occupy the tetrahedral position of the ccp packing respectively. (8a) and the octahedral position (16d), where the tetrahedral lattices 8a, 48f and the octahedral lattice 16c are coplanar to form an interconnected three-dimensional ion channel, which is suitable for free extraction and insertion of lithium ions.
Spinel LiMn2O4 preparation methods include high temperature solid phase method, molten salt impregnation method, co-precipitation method, pechini method, spray drying method, sol-gel method, hydrothermal synthesis, etc.
During the charging and discharging process, LiMn2O4 will undergo a phase transition from cubic to tetragonal, resulting in serious capacity degradation and low cycle life. At present, researchers have improved its electrochemical performance by doping other metal atoms (Co, Ni, Cr, Zn, Mg, etc.) whose radius and valence are similar to that of Mn, and the effect is obvious. But in general, the addition of these doping elements should not be too much, too much dopants will significantly reduce the capacity of the battery. Secondly, adding an excessive amount of lithium salt to the stoichiometric LiMn2O4 can also improve the stability of its crystal structure.
The M2O4 framework in spinel LiM2O4 (M=Mn, Co, V, etc.) is a coplanar three-dimensional network of tetrahedrons and octahedrons that facilitate the diffusion of Li+. Its typical representative is LiMn2O4. Because it is easy to lose oxygen during the heating process and produce oxygen-deficient compounds with poor electrochemical performance, the preparation of high-capacity LiMn2O4 is more complicated. The relationship between the structure and performance of spinel type, especially doped LiMn2O4, is still in the future for lithium ion batteries. The direction of electrode material research.
Like LiCoO2, layered LiMnO2 has a layered structure of α-NaFeO2 with a theoretical capacity of 286mA·h/g. It is stable in the air and is a potential cathode material. Its preparation methods are also diverse. For example, the layered LiMnO2 prepared by the high-temperature solid phase method is charged and discharged at 2.5~4.3V, and the reversible capacity can reach 200mA·h/g. After the first charge, the orthorhombic LiMnO2 will be transformed into a spinel type. LiMn2O4, so the reversible capacity is very poor.
In addition to the transition metal oxides mentioned above as cathode materials for lithium-ion batteries, the hot cathode materials currently being studied are the multi-acid ion system LiMXO4, Li3M2 (XO4) 3 (where M=Fe, Co, Mn, V, etc.; X=P , S, Si, W, etc.).
Since it was reported in 1997 that lithium ions can be reversibly deintercalated in LiFePO4, olivine-type LiMPO4 materials with ordered structures have received widespread attention. Valence in the United States has applied similar materials to the company’s polymerization In the battery.
Lithium iron phosphate (LiFePO4) has a regular olivine crystal structure and belongs to the orthorhombic system (Pmnb). There are four LiFePO4 units in each unit cell. The unit cell parameters are a=0.6008nm, b=1.0324nm and c=0.4694nm.
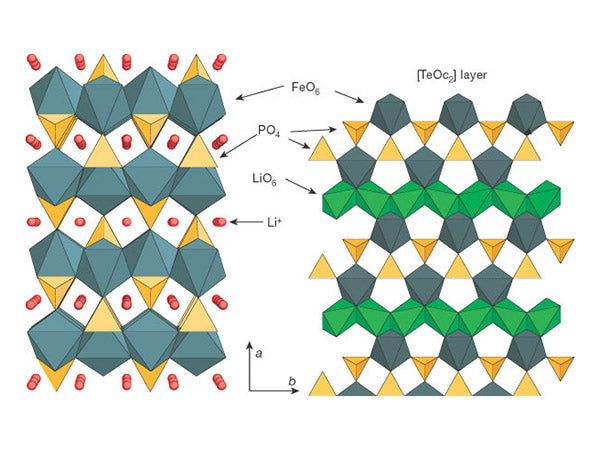
Figure 1 is a schematic diagram of the three-dimensional structure of LiFePO4.
In LiFePO4, the oxygen atoms are arranged in a slightly distorted hexagonal close-packed manner, and Fe and Li are respectively in the center of the oxygen atom octahedron, forming FeO6 octahedron and LiO6 octahedron. The alternate arrangement of FeO6 octahedrons, LiO6 octahedrons and PO4 tetrahedrons forms a layered scaffolding structure. On the bc plane, adjacent FeO6 octahedrons are connected by an oxygen atom sharing a vertex to form an FeO6 layer. Between the FeO6 layers, adjacent Li0 and octahedrons are connected to form a chain in the 6 direction by two oxygen atoms sharing the edge. Each PO, tetrahedron and FeO6 octahedron share two oxygen atoms on the edge, and at the same time share the oxygen atoms on the edge with two LiO6 octahedrons.
The theoretical specific capacity of pure phase LiFePO4 olivine is 170mA·h/g, and the actual specific capacity can reach about 160mA·h/g. The stable olivine structure makes LiFePO4 cathode material have the following advantages:
①Higher theoretical specific capacity and working voltage, 1mol LiFePO4 can deintercalate 1mol lithium ions, and its working voltage is about 3.4V (relative to Li+/Li);
②Excellent cycle performance, especially high temperature cycle performance, and increasing the use temperature can also improve its high-rate discharge performance;
③Excellent safety performance;
④Higher tap density (3.6mg/cm3), higher mass and volume energy density;
⑤The world's iron resources are rich, inexpensive and non-toxic. LiFePO4 is considered an environmentally friendly cathode material.
Nevertheless, LiFePO4 cathode material also has its shortcomings. The material has low ion and electronic conductivity, Fe2+ is easily oxidized to Fe3+ during the synthesis process, and requires relatively pure inert atmosphere protection and other working conditions. At present, LiFePO4 The difficulty of the research is that the synthesis process is difficult, and the high-rate charge and discharge performance of the electrode material is poor.
The most basic condition as a positive electrode material for lithium-ion batteries is that Lit can be reversibly extracted and embedded from the material structure during the charging and discharging process of the battery under the premise of a stable structure. Many phosphates have a structure similar to Na fast ion conductor (NASICON, sodium super ion conductor). In this type of compound, there is enough space to conduct alkali metal ions such as Na+ and Li+, and the most important point is that Compounds have a much more stable structure than transition metal oxides, and they have exceptional stability even when the molar ratio of Li+ to transition metal atoms is greater than 1. Li3V2(PO4)3 is such a compound with NASICON structure.
Li3V2(PO4)3 is a kind of NASICON structure compound. The compound has two crystal forms, namely Trigonal/Rhombohedral and Monoclinic, as shown in Figure 2(a) and (B) Shown.
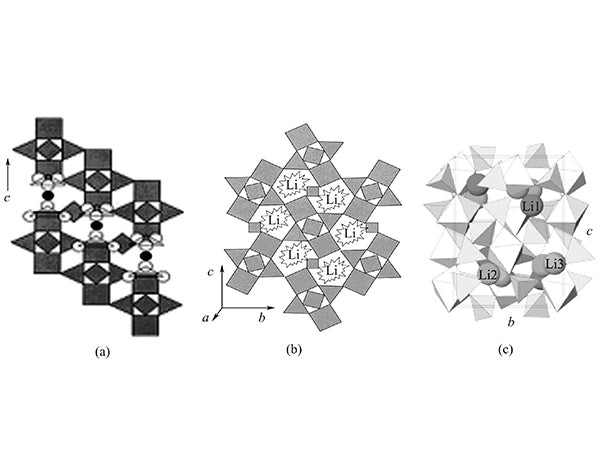
Figure 2 The structure of two different crystal forms of Li3V2(PO4)3
In Li3V2(PO4)3, PO4 tetrahedrons VO6 form a three-dimensional framework structure by sharing vertex oxygen atoms. Each VO6 octahedron has six PO4 tetrahedra around, and each PO4 tetrahedron has four VO6 octahedrons around it. In this way, A2B3 (where A=VO6, B=PO4) is used as the unit to form a three-dimensional network structure. Each single crystal consists of four A2B3 units, and there are a total of 12 lithium ions in the unit cell. Figure 2 (c) shows a three-dimensional view of the monoclinic system Li3V2(PO4)3.
Among the above two crystal types, only the orthorhombic system is a compound of the NASICON structure. In the orthorhombic system Li3V2(PO4)3, the A2B3 units are arranged in parallel [Figure 2(a)], while in the monoclinic system The A2B3 units in Li3V2(PO4)3 are arranged in a B shape [Figure 2(b)], which reduces the space occupied by the guest lithium ion insertion. There are three phases in both of these two crystal forms, mainly because there are different phase transitions at different temperatures. It is α phase at low temperature, β phase at medium temperature, and γ phase at high temperature. The transformation between phases is reversible. The phase transition of α-β and β-γ is only due to the distribution of lithium atoms in the occupied positions. The difference, especially the transition of β-γ phase is a transition from ordered to disordered phase. Li3V2(PO4)3 transforms from α phase to β phase at 120℃, and transforms from β phase to γ phase at 180℃.
The positive electrode of lithium ion battery is usually mixed with active material, such as LiCoO2, LiNixCo1-xO2 or LiMn2O4, conductive agent (such as graphite, acetylene black) and adhesive (such as PVDF, PTFE), etc., and stir it into a paste. , Coat evenly on both sides of the aluminum foil, the thickness of the coating is 15-20μm, dry under a nitrogen stream to remove the organic dispersant, then press it into a shape with a roller press, and then cut it into a pole piece of the specified size as required.















