Main content:
1.Primary battery overview
Primary batteries, batteries, and fuel cells belong to the family of electrochemical power sources.
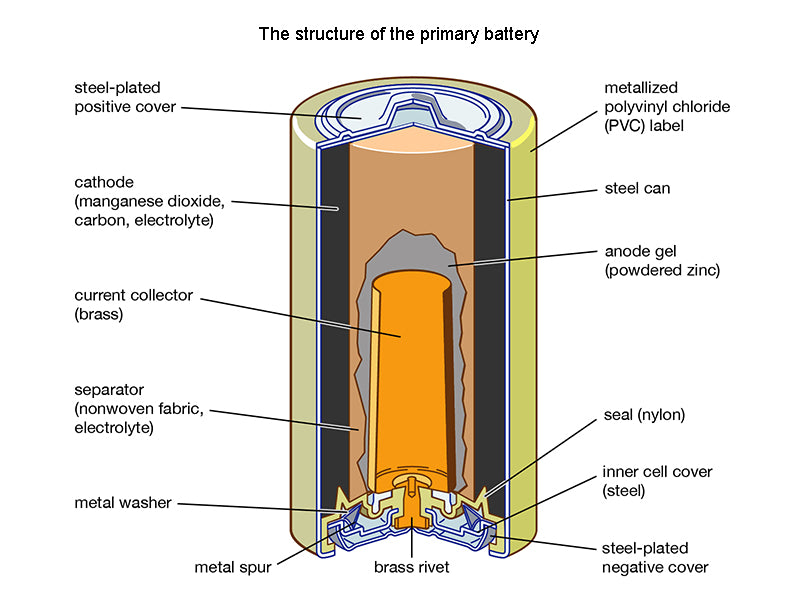
Primary batteries, also considered disposable power sources, are not rechargeable like electrochemical batteries. The amount of power a primary battery can deliver is determined during its manufacture (no charging or other preparation is required before use). Also, it is impossible for a primary battery to return to its original state after being discharged.
2.Battery knowledge
Storage battery, also known as secondary battery, the main difference between storage battery and primary battery is that it can undergo a reversible reaction after being discharged, and can be restored to the original state by charging. Electrochemical battery, a battery is a "reversible" power source that can store electrical energy in the form of chemical energy, and then release the stored electrical energy through reversible transformation at any time as needed. A common feature of electrochemical cells is that they generate electricity by electrochemical reactions between two electrodes immersed in an electrolyte. One of the electrodes is the cathode that absorbs electrons and the oxidant is reduced; the other electrode is the anode that releases electrons and the reducing agent is oxidized.
A battery pack is a module formed by combining some identical battery cells in series and parallel.
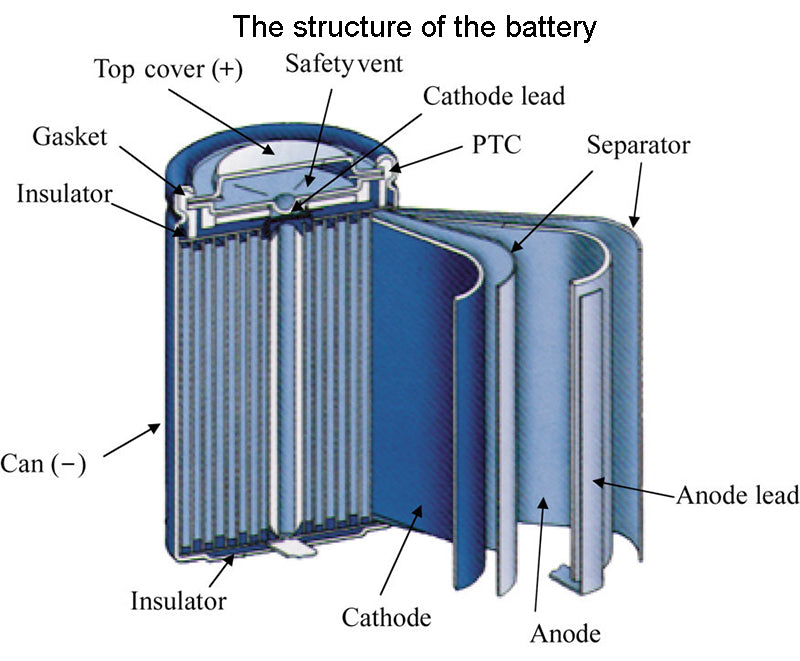
Battery capacity, that is, electricity, usually in Ampere-hours (A h), refers to the amount of electricity that a battery can release during a discharge cycle.
The capacity of the battery is a function of the discharge rate, and the rated capacity of the battery is usually calculated after 10h of discharge (ie, the discharge rate is C/10).
When the discharge rate of the battery is higher than C/10, the amount of electricity that can be released is reduced.
When the discharge rate of the battery is lower than C/10, the amount of electricity that can be released increases.
The unit of discharge current of the battery is ampere, which is usually expressed as a fraction of the ampere-hour capacity (A h) (such as C/100).
For example, a battery with a capacity of 100A·h is discharged at a rate of C/10 (10A) and can be continuously discharged for 10h. If the discharge rate is C/5, the battery capacity will drop to 80A·h, and if the discharge rate is C/100 (1A), the battery capacity will increase to 140A·h.
The capacity of a battery is also a function of its temperature and varies with temperature.
The Faradaic efficiency of a battery is the ratio of its discharge capacity (QD) to its charge capacity (QC), i.e. ηq = QD /QC.
The energy efficiency of a battery is the ratio of the discharge energy to the charge energy in W·h. Energy efficiency depends to a large extent on the charging and discharging technology used and the application environment. Since W·h and A·h are different in the measurement point of charging and discharging electricity, energy efficiency is generally lower than Faradaic efficiency.
The self-discharge rate of a battery refers to the monthly average relative capacity loss of battery capacity at a specific temperature. The self-discharge rate is a technical parameter that reflects the internal characteristics of the battery, and this parameter is usually calibrated at a temperature of 20 °C.
The internal resistance of the battery is generally very small (on the order of a few milliohms) and is inversely proportional to the capacity of the battery. In most cases, the internal resistance of power-type energy storage is lower than that of energy-type energy storage. The low internal resistance of the battery brings a problem to its application. When the two poles of the battery are accidentally connected through a conductive object, since the conductor itself has almost no resistance, the total resistance in the power circuit is very low, resulting in a huge current. A battery that has a short circuit will quickly fail and cannot be used any longer. Of course, the short-circuit test is also one of the standardized tests that must be passed to ensure that the equipment is qualified.
When the battery is in a floating state, the state of charge of its two electrodes is limited by the terminal voltage and tends to balance.
The life of the battery is directly related to its working environment. When used for energy-buffering energy storage, the life of the battery basically depends on the number of charges and discharges and the depth of charge and discharge.
The following figure is a schematic diagram of the operation principle of a rechargeable battery.
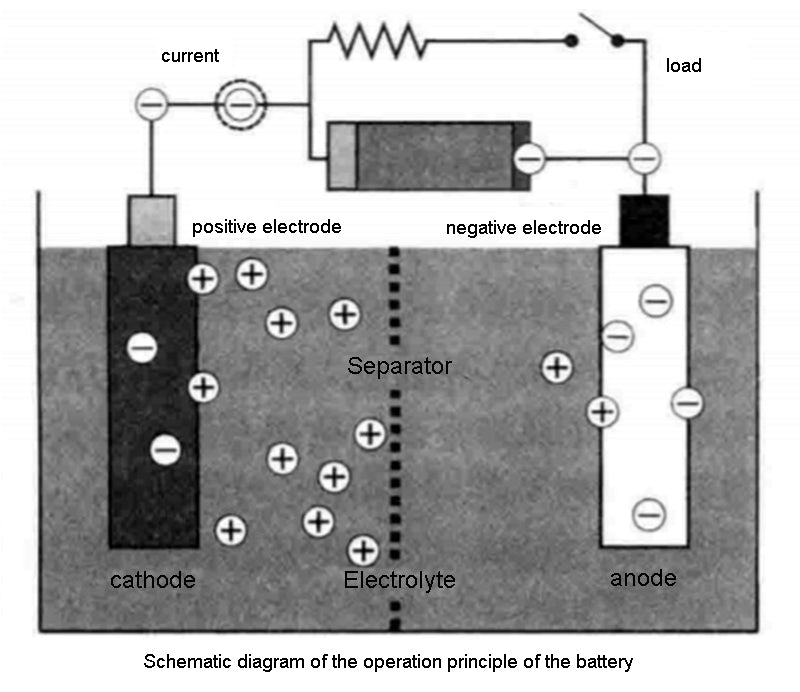
Therefore, both storage batteries and primary batteries belong to electrochemical energy storage systems that release chemical energy (expressed in W·h) in the form of electricity through electrochemical reactions. The term battery is often used to describe the combination of a group of single battery cells (generally referred to as rechargeable batteries). Regardless of the type of technology used, batteries have two basic performance metrics.
1) Specific energy (unit: W·h/kg), which represents the energy that can be stored by a unit mass of battery; power supplied).
2) Cycle life, the unit is the number of charge and discharge cycles, indicating the life of the battery. The cycle life of the lithium-ion battery can reach 4000~6000 times. We often measure the life of the battery by the number of discharges higher than 80% of the rated capacity. This value of 80% is often used in the mobile application of the battery. . It can be seen that, with different applications, the measurement basis of battery life can be changed accordingly.

















