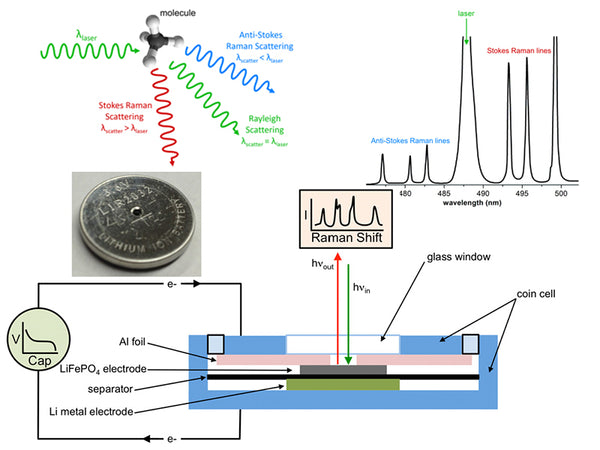
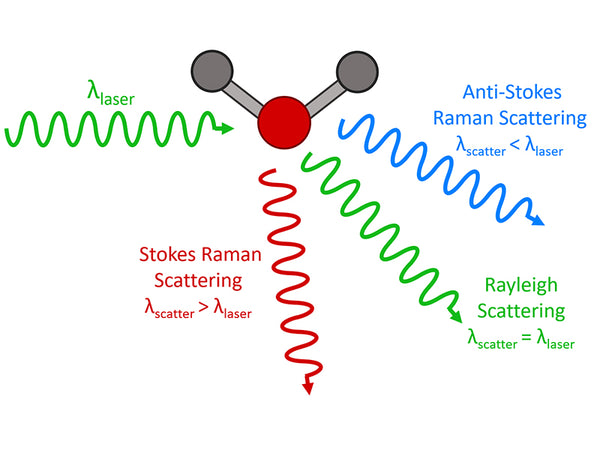
Raman spectroscopy is an analysis method that uses the scattering phenomenon that occurs when a laser beam irradiates a sample to produce a scattering spectrum with a frequency different from that of the incident light. The incident light with a frequency of ν0 can be regarded as a photon with energy hν0. When the photon collides with a substance molecule, the energy may remain unchanged, so the frequency of the scattered light generated is the same as the frequency of the incident light. Only the movement direction of the photon changes, this kind of elastic scattering is called Rayleigh scattering. In an inelastic collision, energy exchange occurs between photons and molecules. The photon gives part of the energy to the molecule or obtains part of the energy from the molecule, and the energy of the photon will decrease or increase. On both sides of the Rayleigh scattering line, a series of scattered rays that are lower or higher than the frequency of the incident light can be seen, which is Raman scattering. If the molecule was originally in the low energy level E1 state, and the result of the collision makes the molecule transition to the high energy level E2 state, the molecule will gain energy E2-E1, and the photon will lose this part of energy. The frequency of the photon becomes:
ν-=ν0-(E2-E1)/h=ν0-(△E/h)
Namely the Stokes line. If the molecule was originally in the high energy level E2 state, and the result of the collision makes the molecule transition to the low energy level E1 state, the molecule will lose energy E2-E1; the photon gains this part of energy, and the photon frequency becomes:
ν+=ν0+(E2-E1)/h=ν0+(△E/h)
It is the anti-Stokes line.
The difference between the frequency of the Stokes line or the frequency of the anti-Stokes line and the frequency of the incident light, expressed as Δν, is called the Raman shift. The Raman displacement Δν of the corresponding Stokes line and the anti-Stokes line are equal, namely:
△ν=ν0-ν-=ν+﹣ν0=(E2-E1)/h
At room temperature, according to Boltzmann's law of distribution, the number of molecules at low energy level E1 is much more than the number of molecules at high energy level E2, so the Stokes line is much stronger than the anti-Stokes line; The intensity of the Li spectrum is several orders of magnitude higher than the intensity of the Raman spectrum.
Raman spectroscopy
From the above discussion, it can be seen that the frequency shift Δν of Raman scattering has nothing to do with the incident light frequency ν0 (this makes it easy to select an incident light source with an appropriate frequency, such as selecting visible light as the incident light source for Raman spectroscopy), and is related to the molecular structure. That is, the Raman shift △ν is the vibration or rotation frequency of the molecule. The molecules of different compounds have different Raman shifts △ν, the number of Raman spectral lines and the relative Raman intensity, which are the basis for qualitative identification of molecular groups and molecular structure analysis. For the same compound, the Raman scattering intensity has a linear relationship with its concentration.
The Raman spectrum appears in the visible light region, and its Raman shift is generally 4000~25cm-1 (the lowest can be measured to 10cm-1), which is equivalent to the near-infrared to far-infrared spectrum with a wavelength of 2.5~100μm (the longest is 1000μm) The frequency, the Raman effect, corresponds to the transition of the molecular rotational energy level or vibrational rotational energy level. When directly studied by absorption spectroscopy, this transition occurs in the infrared region or the far-infrared region, and the infrared spectrum is obtained. The mechanism of the Raman spectrum and the infrared spectrum is essentially different. Raman spectroscopy is a kind of scattering phenomenon, which is caused by the change of the polarizability of the molecule when it vibrates or rotates (that is, the change of electron cloud in the molecule), while infrared spectroscopy is an absorption phenomenon, which is the dipole when the molecule vibrates or rotates. Caused by moment changes.
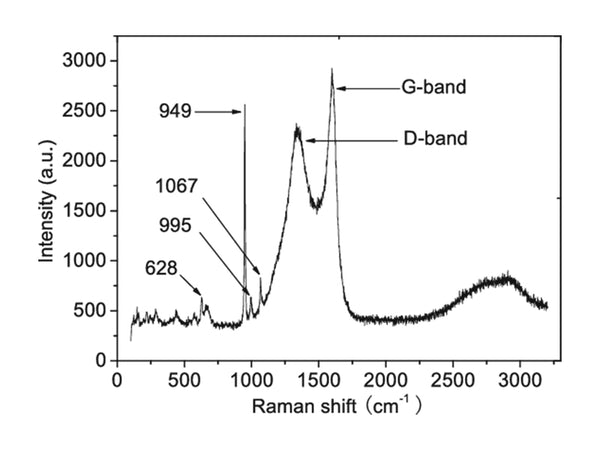
Raman spectra of electrode materials
Raman spectroscopy is derived from the change of molecular polarizability, which is caused by the symmetrical vibration of bonds with symmetrical charge distribution (this kind of bond is easy to polarize), so it is suitable for studying non-polar bonds of the same atom.
Laser Raman spectrum vibration superposition effect is small, the band is clearer, and the double frequency and group frequency are very weak. It is easy to measure the degree of polarization to determine the symmetry of the substance molecule, so it is easier to determine the band fate. There is a certain convenience in the distribution of the spectrum. Raman spectroscopy can directly measure gas, liquid and solid samples, and water can be used as a solvent. It can be used for the research on the stereoregularity, crystallinity and orientation of polymers, and it is also a powerful tool for the analysis of inorganic materials and metal-organic compounds. For inorganic systems, it is much better than infrared spectroscopy. It can be measured not only in aqueous solution, but also metal-coordination bond vibration in complexes whose vibration frequency is in the range of 1000~700cm-1.















