
Since the electrochemical reactions in the battery charging and discharging process can only occur in a specific temperature range, which means that the ambient temperature range of the battery operation is specific, Table 1 gives several typical power batteries, the characteristics and Permissible temperature range for operation.
| Battery Type |
Lead-acid batteries | Ni-MH battery | Li-ion battery |
| Energy density/(W·h·kg-1) | 30~50 | 60~120 | 110~200 |
| Fast charging time/h | 8~16 | 2~4 | 2~4 |
| Voltage/V | 2 | 1.25 | 3.6 |
| Self-discharge rate (room temperature)/% | 5 | 30 | 3 |
| Operating temperature range/℃ | -20~+60 | -20~+60 | 0~45 |
Table 1 Power battery performance parameters
Safety issues such as overheating, combustion, and explosion have always been the focus of power battery research. The generation and rapid accumulation of heat will inevitably cause the internal temperature of the battery to rise, especially when used in a high temperature environment or charged and discharged with a large current, which may cause a violent chemical reaction inside the battery, generating a large amount of heat. If the battery accumulates rapidly inside the battery, the battery may leak, outgas, and smoke. In severe cases, the battery will burn violently or even explode. Whether traditional lead-acid batteries or advanced Ni-MH, Li-ion power batteries, temperature has a very significant impact on the overall performance of the battery. Generally speaking, temperature mainly affects the following performance of power battery:

Figure 1 Electric vehicle thermal safety accident
(1) Electrochemical system operation;
(2) Charge and discharge efficiency;
(3) The rechargeability of the battery;
(4) The power and capacity of the battery;
(5) The reliability and safety of the battery;
(6) Battery life and cycle times.
The temperature increases, the internal resistance of the battery decreases, and the battery efficiency increases. However, the increase in temperature will accelerate the rate of harmful chemical reactions inside the battery, thereby destroying the battery. In general, a 10°C increase in temperature doubles the rate of chemical reactions. When the Ni-MH battery works at 45°C, its cycle life is shortened by 60%; when charging at a high rate, the battery life is reduced by half for every 5°C rise in temperature. The optimum operating temperature range for Ni-MH batteries is 20~40℃; for lead-acid batteries, it is 25~45℃. Ramadass et al. studied the cycle performance of Sony 18650 (capacity 1.8A h) Li-ion battery. The results are shown in Table 2. After the battery was operated for 800 cycles at 25°C and 45°C, the battery capacity decreased by 31%, respectively. and 36%; when the operating temperature is 50°C, the battery capacity decreases by 60% after 600 cycles; when the operating temperature is 55°C, the capacity decreases by 70% after 500 cycles. The results of Sarre et al. showed that the capacity of Li-ion batteries only decayed by 4% after 22 months of cycling at 40°C (80% depth of discharge). Wu et al. fully charged the Li-ion battery and placed it at 25°C and 60°C for 60 days, respectively. The capacity of the battery at room temperature decayed from 800 mA h to 790 mA h, while the capacity of the battery placed at 60°C decreased. decay to 680mA•h. When the capacity decay rate is 30%, the cycle life of the Li-ion battery is 3323 cycles at 45°C and only 1037 cycles at 60°C. For both Ni-MH and Li-ion batteries, the battery life decreases when the temperature exceeds 50°C. Table 3 summarizes the capacity fading of current Li-ion batteries as a function of operating temperature. In general, the optimal operating temperature range of lead-acid batteries, Ni-MH and Li-ion power batteries is 25~40℃, and the temperature difference between battery modules is less than 5℃.
| temperature/℃ | Cycles | Capacity loss/(mA h) |
| 25 |
150 300 800 |
28 38 71 |
| 45 |
150 300 |
27 33 |
| 50 |
150 300 600 |
58 62 95 |
| 55 |
150 300 |
29 81 |
Table 2 Capacity loss of Sony 18650Li-ion battery under different temperature cycles
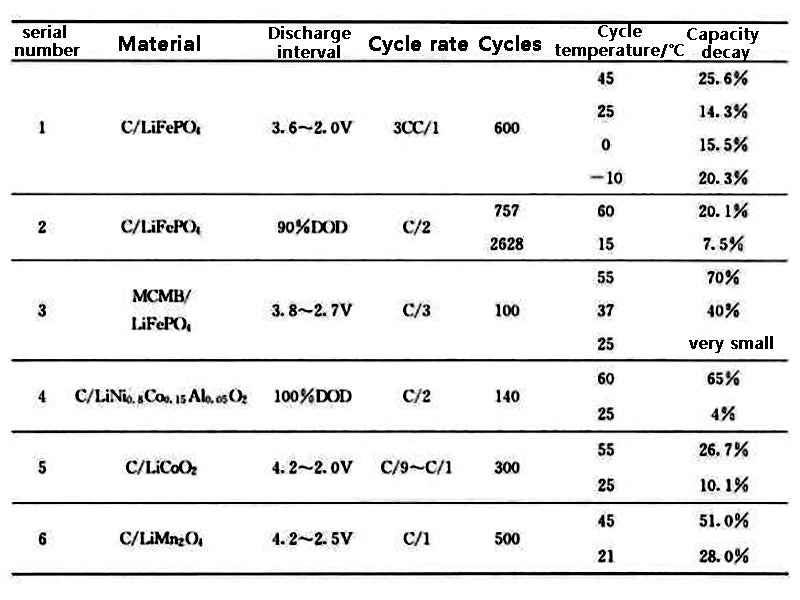
Table 3 Relationship between capacity fading and temperature of Li-ion batteries
During the driving process of the electric vehicle, the discharge current of the power battery fluctuates. When the car starts, accelerates, etc., the current changes greatly and the heat generation is unbalanced. With the development of electric vehicles, the power requirements of the power system continue to increase, and the increase in the demand for fast charging and discharging causes the battery to generate a lot of heat when it is discharged at a high current. The heat generated inside the battery often raises the temperature of the single cell inside the battery module to 100°C, and even reaches 199°C during overcharge, which is 93°C higher than the surface temperature. The resulting high temperature may ignite surrounding flammable materials and cause external combustion of the product, creating a safety hazard. For a single battery, as the battery size increases, the imbalance of heat generation inside the battery becomes more prominent, and the heat generation of the positive electrode reaction is even three times that of other parts. Due to the temperature difference inside and outside the battery, the temperature difference between the inside and outside of the battery, and the limitation of heat dissipation, a very serious unbalanced temperature distribution occurs between the different modules in the battery pack and the individual cells inside the battery module, resulting in the performance mismatch between the single cells, which further leads to The battery module failed prematurely.
The application of power batteries in electric vehicles generally needs to comprehensively consider the impact of temperature on battery performance and cycle life to determine the optimal working range of the battery, and obtain the best balance of performance and life within this temperature range. Heat is released during charging and discharging, regardless of the electrochemical resistance between the various parts inside the battery or the electronic conduction resistance. When the battery is used in power applications, the discharge current is very large, for example, a large current of 10C and 20C (equivalent to several hundred amperes), and a small internal impedance of the battery may cause a large amount of heat to be released. In addition, in the case of low temperature (such as less than 0 °C), the charging and discharging capacity of the battery will be reduced, and the possible reasons include the freezing and solidification of the electrolyte. For some areas, the temperature in winter is often lower than -20 ℃, and the battery cannot be discharged basically, or the depth of discharge is shallow. Figures 2 and 3 show the results of the follow-up survey of three different vehicles, Tesla Model S, Leaf and Volt. It can be seen that under the same operating conditions, the temperature of the battery pack has a greater impact on the cruising range of electric vehicles.
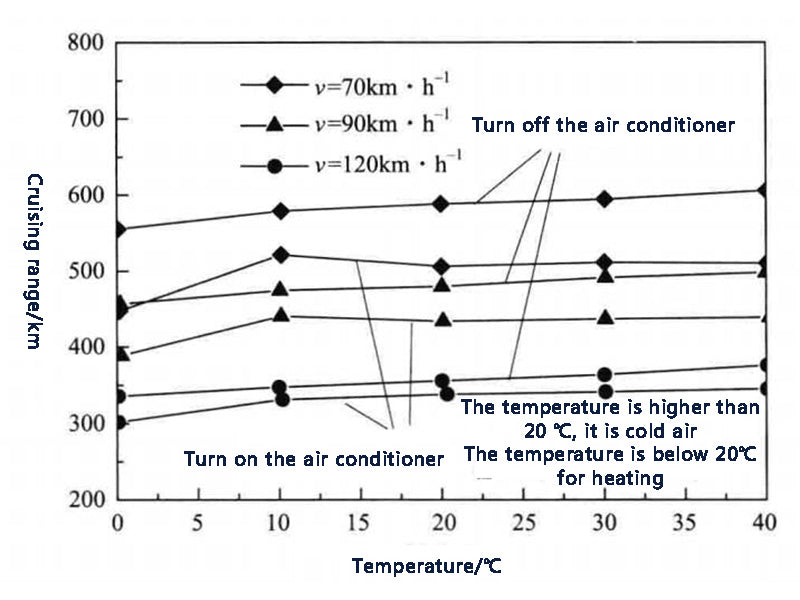
Figure 2 The cruising range of Tesla Model S at different temperatures and working conditions
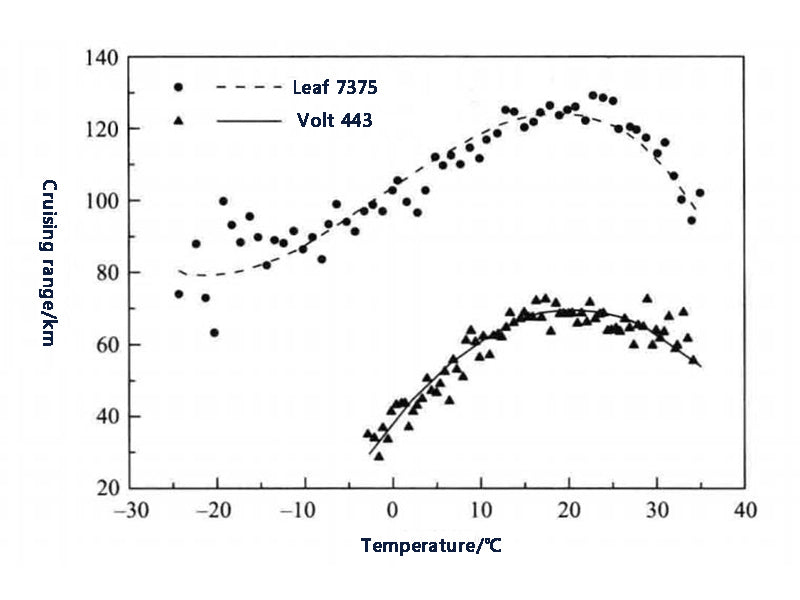
Figure 3 The cruising range of Leaf and Volt at different temperatures
Too high or too low temperature is not conducive to the performance of the power battery. In order to prolong the life of the power battery and improve its electrochemical performance and energy efficiency, a reasonable battery heat management system must be designed to dissipate heat from the battery under high temperature conditions, and heat or heat the battery under low temperature conditions to improve the overall performance of electric vehicles. .
















