Some people think that the only function of plasticizers is to lower the glass transition temperature of the polymer electrolyte, dissolve the lithium salt, and make the polymer amorphous, so that ions and other carriers can move freely in it to improve the conductivity of the electrolyte. Others believe that in addition to plasticizing, the more important role of plasticizers is to associate with ion carriers and make them move faster. So far, most researchers have referred to polymer electrolyte systems containing plasticizers as mixed phase or composite electrolytes. This means that the interaction between the components in the polymer electrolyte is ignored.
The polymer electrolytes represented by PAN, which are blended or plasticized, will be studied, and dozens of PAN-based polymer electrolytes containing plasticizers will be synthesized. The plasticizers used include propylene carbonate PC, ethylene Carbonate EC, dimethyl formamide (DMF), dimethyl sulfoxide (DMSO), γ-butyrolactone (γ-BL) and their mixtures, etc. Through research, the room temperature conductivity of polymer electrolytes can reach 2.5×10-3S/cm, and these electrolytes show good compatibility with metallic lithium.
Research on the commonly used solvents EC, DMF and DMSO containing different concentrations of LiClO4, found that their Raman and infrared spectra changes. Although these systems have different details, their common spectral characteristics can be summarized as follows. The peak positions with the most obvious changes in the spectrum are related to the C=O group (EC and DMF) or the S=O group (DMSO). Therefore, the spectral changes associated with these peaks undoubtedly indicate the interaction between LiClO4 and the solvent, or more precisely, reflect the relationship between the lithium ion and the C=O or S=O group in the solvent molecule. The interaction between them.
Figure 1 is the Raman spectrum (715cm-1) of EC ring bending vibration mode as a function of solution concentration. Although the interaction mainly occurs on the carboxyl group and S=O group, due to the electronegativity of these nitrogen and oxygen atoms, it is not excluded that some lithium ions can interact with the oxygen atoms on the EC ring or with the nitrogen atoms on the DMF. Possibility of interaction.
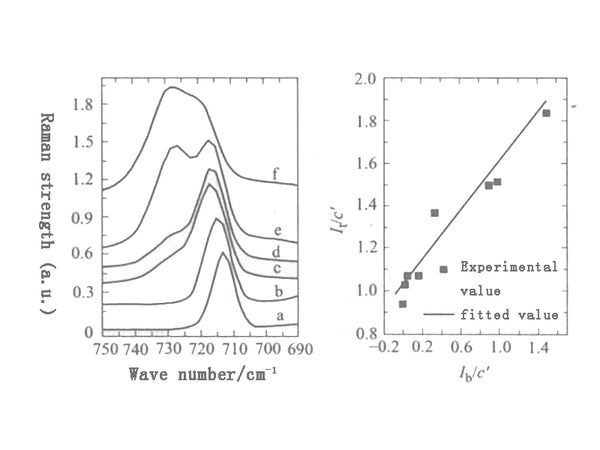 Figure 1 Raman spectra of different molar ratios of LiClO4 and EC a-0 (pure EC); b—0.008; c—0.04; d—0.09; e—0.21; f—0.35
Figure 1 Raman spectra of different molar ratios of LiClO4 and EC a-0 (pure EC); b—0.008; c—0.04; d—0.09; e—0.21; f—0.35
In addition, the results of infrared spectroscopy and Raman spectroscopy both show that all the Raman or infrared active vibration modes of perchlorate anion such as v1 (A1, 930cm-1), v2 (E1, 457cm-1), v3 (F2, 1060cm-1) And v4 (F2, 624cm-1) split, corresponding to the decrease of anion symmetry and the formation of different ion associations, such as ion pair separated by solvent (Li+-solvent-ClO4-), contact ion pair (Li+ClO4- ) And multi-ion clusters [(Li+ClO4-)n, n≥2]. The increase of these aggregates will lead to a decrease in the carrier concentration in the solution and an increase in the viscosity of the solution, reducing the mobility of the carriers and reducing the conductivity of the electrolyte.
Determining the association number of lithium ions in solution helps to understand the transport mechanism of lithium ions and design new electrolytes. Taking the strength of the ring stretching vibration mode of the EC molecule at 1230 cm-1 as the internal standard, the content of free and associated solvent molecules can be calculated by quantitatively studying the change of the EC molecule's ring bending vibration mode with the concentration of lithium perchlorate. And the number of associations of lithium ions in the solution.
Define cf and cb as the concentration of free and associated EC molecules in the electrolyte, and If and Ib are the relative intensities of the corresponding vibration peaks (ie If=I715/I1230; Ib=I725/I1230), we can get The following relationships:
If=cfJf, Ib=cbJb —— (1)
Among them, Jf and Jb are the coefficients related to the molecular species and its scattering conditions, and the total relative intensity is:
It=If+Ib=cfJf+cbJb=(1-Jb/Jf)If+cJb —— (2)
Where c=cf+cb is the concentration when the solvent is converted into the amount of substance (for EC, c=11.36mol/kg)
The above formula (2) shows that the relationship between It and If is linear, with a slope of 1-Jb/Jf and an intercept of cJb. There is basically a linear relationship between It and If. Fitting can get:
1-Jb/Jf=0.238, cJb=1.386 —— (3)
therefore:
Jf=0.160, Jb=0.122 —— (4)
Define the lithium ion association number (equivalent to the average coordination number of lithium) as:
ns=cb/cLi+=Ib/(cLi++Jb) —— (5)
According to formula (5), it can be considered that the association number of lithium ions in EC is 6, which is within the most possible range of ion association number (generally considered that the association number of lithium ions is 4, 5 or 6) It is worth noting that the calculated ns value decreases with the increase of the solution concentration. This is because it is assumed that all LiClO4 is dissolved by EC, so cLi+ is used instead of CLiClO4. In other words, the cLi+ in formula (5) should be the concentration of free lithium ions cLi+ instead of the concentration of LiClO4, CLiClO4. Due to the appearance of ion pairs, cLi+ is smaller than CLiClO4, and the difference between the two increases as the concentration of the solution increases, and the two are equal only when the concentration of the solution is very low.
Analyzing the Raman spectra of EC and PAN and their mixtures, we can see the significant influence of PAN on the Raman spectra of EC at different relative contents. The spectral peak with the most obvious change in spectral characteristics is the C=O stretching vibration mode of EC (1762cm-1). Its intensity has changed from the moderate intensity in pure EC to the weak peak in EC/PC mixture, and its position is also higher in wave number. The segment moved 10cm-1 (Figure 2). In addition, the EC ring stretching vibration mode has moved from 1059cm-1 to 1072cm-1, accompanied by a decrease in relative strength and an increase in line width. These spectral changes indicate that there is a strong interaction between EC and PAN. On the other hand, the effect of EC on the spectral changes of PAN is not obvious. One possible explanation is that the cyano bond is much stronger than the carbon bond, and the same intensity of the interaction causes different spectral changes between the two. This interaction may occur through the dipole interaction between the carbon group of EC and the cyano group of PAN.
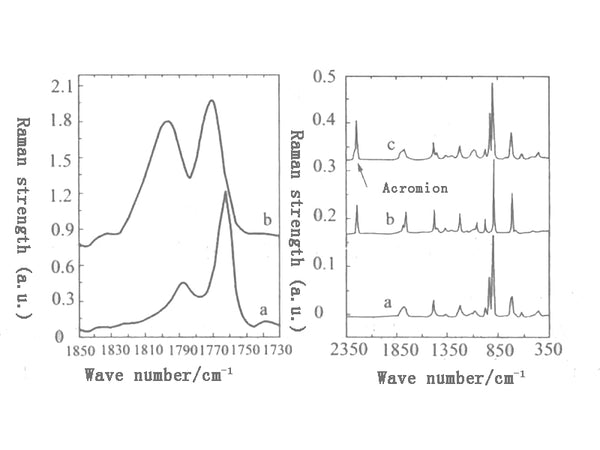
Figure 2 EC with different molar ratios: C=O stretch Raman spectrum of LiCIO4 into a—pure EC; b—pure LiCIO4; c—nEC: nLiClO4=3:21
When DMSO is added to PAN, when the molar ratio of PAN:DMSO is very low, the spectral change of DMSO is very obvious. A new peak appeared at 1070 cm-1 (corresponding to DMSO monomer) while the intensity at 1013 cm-1 and 1030 cm-1 (corresponding to linear diploid and DMSO aggregates of DMSO, respectively) decreased. The line width of S=O bending vibration mode at 303cm-1 increases with the increase of PAN content in the mixture. Obvious spectral changes were also observed in other vibration modes, such as the CSC stretching vibration mode of DMSO. These indicate that the influence of PAN on DMSO is realized by the S=O group on DMSO and the dissociation of linear diploid in DMSO. This effect is not observed when the content of PAN is relatively low, but when the molar ratio of PAN:DMSO reaches 0.8, the Raman scattering cross section of the cyano stretching vibration mode (2240cm-1) becomes smaller.
Figure 3 is the Raman spectrum of the N=C-O group deformation vibration mode of DMF when PAN is dissolved in dimethylformamide (DMF)/LiClO4 solution. The Raman spectrum of this vibration mode in the DMF/LiClO4/PAN system is very similar to the Raman spectrum in the DMF/LiClO4 system. At any comparable concentration, the addition of PAN will not cause the Raman spectrum of the vibration mode to change. This shows that PAN does not affect the interaction between DMF and Li+, and similar phenomena can also be observed in the DMSO/LiClO4/PAN system.
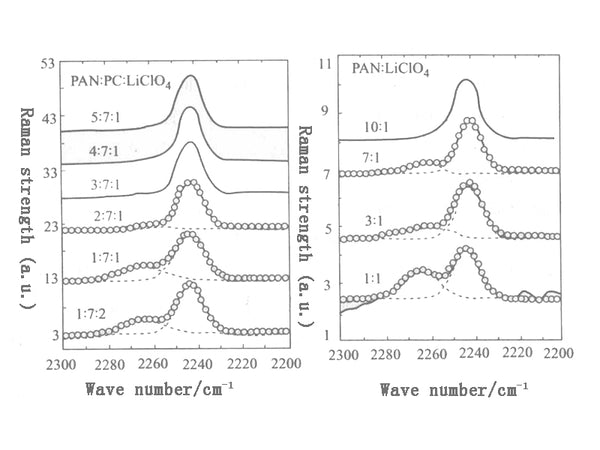
Figure 3 PAN dissolved in pure PC, LiCIO4 and different PC: LiCIO4: PAN molar ratio N-C-O group deformation vibration in DMF solution
When PAN is added to propylene carbonate (PC), the vibration mode of ring deformation changes significantly before and after comparison. Adding PAN to the solvent makes the interaction between Li+ solvent (or plasticizer) insignificant. In the PC/LiClO4 system, the 722cm-1 peak split from the 712cm-1 peak of the ring deformation vibration of PC becomes obvious when the PC/LiClO4 molar ratio is 10:1. But in the PC/LiClO4/PAN system, when the concentration of PAN is relatively large (that is, when the molar ratio of PC:LiClO4:PAN changes from 7:1:3 to 7:1:5), even at very high lithium salts Concentration (PC/LiClO4=7:1, molar ratio), this component is still not observed, indicating that the splitting of the ring deformation vibration mode strongly depends on the content of PAN in the PC/LiClO4 solution. If the PAN content is high, the interaction between lithium ions and the solvent can only be observed at a high PC:LiClO4 concentration. On the contrary, if the content of PAN is low, even at a very low PC:LiClO4 molar ratio, it is easy to see the interaction between lithium ions and the solvent.
Similar to the EC/LiClO4/PAN system, the interaction between lithium ions and PAN can also be observed in the PC/LiClO4/PAN system. When the mole fraction of LiClO4 is much smaller than that of PAN, the peak shape of the stretching vibration mode of the cyano group is almost unchanged before and after PAN is added. When the mole fraction of PAN in the electrolyte is further reduced, the peak shape of 2240 cm-1 starts to deviate from the original symmetrical shape.
The Raman spectrum of DMF/LiClO4/PAN electrolyte with a molar ratio of 10:1:3~10:3:3 to 10:2:4 has also been measured, but DMF/LiClO4/PAN is in this range The change of the molar ratio of the cyano group does not affect the change of the peak shape and peak position of the cyano stretching vibration mode. This indicates that there is no detectable strong interaction between the lithium ion and the cyano group. These results are consistent with the results seen in the DMSO/LiClO4/PAN system.
Summarizing the above results and discussions, it can be seen that the polymer body and the plasticizer interact with each other in terms of association with lithium ions. On the one hand, the addition of PAN to PC/LiClO4 inhibits the interaction between lithium ions and PC molecules. Whether the interaction between lithium ions and PAN can be observed is not only related to the content of plasticizer, but also to the polymer The content of PAN in the electrolyte is related. On the other hand, within a wide range of the molar ratio of DMF (or DMSO), PAN only has a weak effect on the interaction between Li-DMF, and no interaction between lithium ions and PAN is observed. There is a competition between the association of PAN with lithium ions and the association of plasticizers with lithium ions.
The three-component polymer electrolyte (plasticizer, polymer, and lithium salt) is far more complex than the two-component electrolyte system (such as a solid electrolyte system composed of polyethylene and lithium salt). For example, pour a gel polymer electrolyte composed of LiClO4, PAN and a plasticizer (such as PC) on a glass plate and heat it at 120-140°C for 6 hours to remove most of the plasticizer. The sample was then transferred to the vacuum chamber (0.1 Pa) of the glove box and stored there for 7 days to completely remove the residual plasticizer. Finally, the LiClO4-PAN film with the plasticizer removed was peeled off and used for spectrum measurement. The resulting Raman and infrared spectroscopy analysis showed that there were no detectable plasticizer residues in the sample.
The order of the competitiveness of the three polar materials of DMF, PC and PAN to form ion associations with lithium ions is: DMF>PC>PAN. Since the competitiveness of PAN is much weaker than that of DMF, when PAN and DMF When coexisting, it is difficult to observe the association between lithium ion and PAN. Most of the lithium ions have been associated with DMF. But in the PC/LiClO4/PAN polymer electrolyte system, it is easy to observe this association between lithium ions and PAN. When the plasticizer is removed, there is no plasticizer to compete with PAN for lithium ions. In this case, even if the mole fraction of LiClO4 is very low (LiClO4:PAN<1:10), it is easy to observe the association between lithium ions and PAN.
It can be seen from Figure 4 that when the mole fraction of LiClO4 is relatively high, the two Lorentzian components located at 933cm-1 and 954cm-1 can well decompose the Raman peaks of the vibration mode. contour. The peak at 933 cm-1 comes from the undisturbed ClO4-anion. The peak at 954 cm-1 can be attributed to the polyion association [(Li+ClO4-)n] in the LiClO4/PAN system, but this is inconsistent with the observations about lithium ion associations in LiClO4 solution. Generally, in the liquid perchlorate solution, the polyion association always appears behind the ion pair separated by the solvent and the contact ion pair (at higher lithium salt concentration). One possible explanation is that the disturbance of LiClO4 by PAN is smaller than that of ordinary solvents of LiClO4, and the component at 954cm-1 comes from the recrystallization of LiClO4. This may be why PAN is not as competitive as other plasticizers (such as PC and DMF) in association with lithium ions. The IR spectrum of the activated v1 mode is consistent with the Raman spectrum (not shown).

Figure 4 Raman spectrum of CIO4-ion vibration mode with different molar ratios of LiCIO4/PAN (with the plasticizer removed) a—1:7:b—1:4:c—1:1
The conductivity of the electrolyte depends on the concentration of carriers and the mobility (mobility) of carriers. The degree to which ion pairs dissociate and become carriers is determined by the dielectric constant of the plasticized polymer electrolyte. When the concentration of LiClO4 in the LiClO4/solvent system is very low, most of the lithium salt can be dissociated, and the ion conductivity of the solution increases almost linearly with the increase of the lithium salt concentration. However, as the concentration of lithium salt increases, ion pairs appear in the solution, and ion association begins to dominate, resulting in a decrease in the concentration of free carriers and thus a decrease in the conductivity of the solution. The number of lithium ions around EC molecules in the EC/LiClO4 solution can be as high as 6.
In the LiClO4/PAN electrolyte system (with the plasticizer removed), lithium ions associate with the PAN molecular chain through comb-shaped cyano groups. One lithium ion can associate with four cyano groups. Since the interaction between perchlorate ions and PAN is very weak, they exist as lone ion carriers after being separated from lithium ions. When the content of LiClO4 is further increased, excess lithium ions (relative to the association number 4 with PAN) will combine with ClO4- and recrystallize. Because lithium ions are tightly bound by the nitrogen atoms on the PAN side chain due to the association at room temperature (without plasticizers, the glass transition temperature of PAN is as high as 169°C), the ionic conductivity of the LiClO4/PAN system will be very Low, and the main contribution to the conductivity comes from the relatively free perchlorate ions, that is, the ion migration number t->>t+.
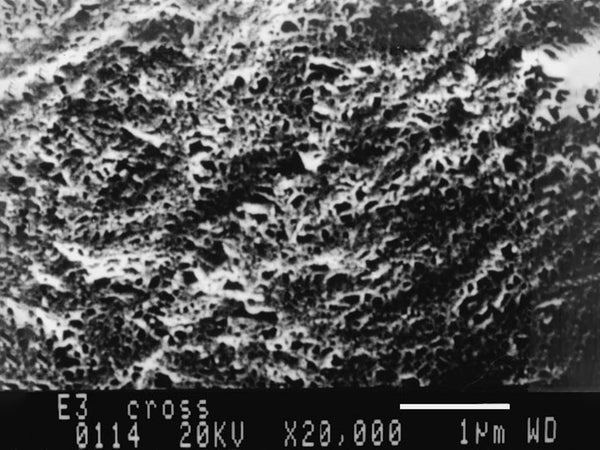
Figure 5 SEM picture of PAN-based polymer electrolyte cross section
When both LiClO4 and PAN are dissolved in the plasticizer, the coordination bond between the lithium ion and PAN will be broken due to the strong competitiveness of the plasticizer for lithium ions. In the polymer electrolyte plasticized with PC or EC, the dissociation effect of the plasticizer becomes more obvious with the increase of the plasticizer cost. The dissociation effect becomes more obvious because the polymer electrolyte plasticized by the plasticizer DMF or DMSO has a stronger ability to compete and associate than EC or PC. In such an electrolyte, as long as the LiClO4/PAN mixture can be dissolved in the plasticizer, the PAN molecules associated with lithium ions cannot be detected by Raman spectroscopy and infrared spectroscopy. Therefore, in such a system, even if there are PAN molecules associated with lithium ions, their number must be very small. In addition to the disassociation of the plasticizer molecules to Li+-PAN, there is also a mutual repulsion between the polymer molecules and the dipoles of the plasticizer molecules.
It has been repeatedly proved that the mobility of bond ions in the amorphous region of the polymer electrolyte composed of PEO-lithium salt is the highest. 7Li NMR studies have shown that there are at least three different regions in PAN-based gel polymer electrolytes: ①Lithium ions move in the gel state, which is the part that contributes the most to the ionic conductivity of the polymer electrolyte; ②Always Lithium ions that move along the PAN segment and ③ lithium ions that associate with plasticizer molecules. In addition, the analysis of the relationship between the line width of the 7Li NMR spectrum and the temperature shows that most of the movement of lithium ions is between the long range and the short range. As for the long-range movement of lithium ions, it is very likely that lithium ions move along the side chain of PAN, jumping from one position to another. According to Raman spectroscopy, infrared spectroscopy and X-ray photoelectron spectroscopy XPS, this position is most likely provided by the cyano group on the PAN. According to polymer physics, the length of PAN molecular chain is about a dozen or so PAN basic units [-CH2-CH(CN)-], and the length is about several nanometers. When the polymer main chain moves in the form of segment motion, it is easy to understand that the movement of lithium ions with the PAN segment in the solid state is long-range. If lithium ions move in a liquid medium, their movement will be strongly affected by the Brownian motion of solvent molecules, so it is short-range. Gel is a state between liquid and solid. Therefore, the movement of lithium ions in the gel state is also between the long-range movement in the solid state and the short-range movement in the liquid state. Therefore, the lithium ion is in the gel state. The movement in the polymer electrolyte contributes the most to the conductivity of the polymer electrolyte.
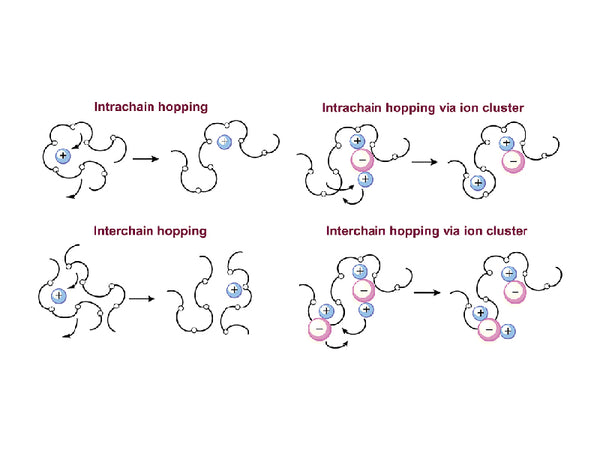
Figure 6 Mechanism of ion transport in PEO
By associating with the side chain of the polymer, the movement of lithium ions is very similar to the movement of the chain segments in the PEO salt electrolyte, so their movement is long-range. However, at room temperature, the ion conductivity of PAN polymer electrolyte is not mainly derived from the contribution of lithium ions along the chain segment, because the movement of the PAN segment is very slow (compared to the gel state). In this regard, it is very different from the case of PEO polymer electrolytes without plasticizers. In PEO polymer electrolytes, segment movement is the most important contribution to the conductivity of the electrolyte. Of course, when the conductivity of the polymer electrolyte is increased, the movement of the chain segment will be accelerated, so the total conductivity of the polymer electrolyte is closer to that of the liquid electrolyte.
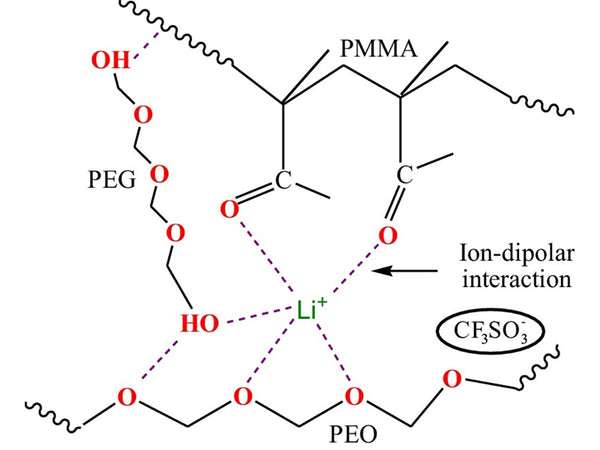
Figure 7 PEO polymer electrolyte
In PAN-based polymer electrolytes, the migration number of anions (approximately 0.64) is generally greater than the migration number of cations (approximately 0.36). The movement of ClO4-anion in gel polymer electrolyte is similar to that in liquid; because the interaction between anion and PAN is very weak, the anion does not move along the polymer chain, but is provided in the PAN chain Jump from one position to another directly outside of the channel. On the one hand, the weak solvation between the anion and the plasticizer molecule allows the anion to move at a faster speed than the cation. On the other hand, since the viscosity of the gel electrolyte is greater than that of the liquid electrolyte, the anion has a huge volume Make it more severely hindered than when it moves in liquid electrolyte. These two effects coexist, and as a result of their interaction, the movement of anions in the gel polymer electrolyte is slower than in the liquid electrolyte.
Figure 8 Thermogram of PAN-based polymer electrolyte
Based on the above discussion on the transport mechanism of anions and cations, the role of plasticizers in PAN-based polymer gel electrolytes is summarized as follows:
①Reduce the glass transition temperature of the polymer electrolyte and dissociate the crystalline state of the polymer, which will increase the mobility (mobility) of the polymer segment and help the transport of carriers that move with the polymer segment. Transport (the movement of ions in the solid electrolyte);
②Dissolve the electrolyte salt and provide carriers for the polymer electrolyte;
③Through the interaction between the dipole and the polymer molecule, the polarity of the polymer and its own is improved, which in turn promotes the dissociation of the lithium salt in the polymer electrolyte;
④ Break the coordination bond between the lithium ion and the polymer, so that more lithium ions move in the gel state instead of in the solid phase.
According to the role of the plasticizer in the polymer electrolyte, the selection criteria for the polymer electrolyte plasticizer are as follows:
① Compatibility with polymers and electrode materials;
② Thermodynamically stable;
③Low viscosity;
④High dielectric constant;
⑤Low melting point and high boiling point;
⑥Non-toxic and easily available;
⑦The plasticizer should have strong competitive ability to dissociate the association between lithium ion and polymer (Li+-PAN), but this competitive ability should not be too strong, otherwise it will generate a strong Li+-plasticizer -ClO4- and other associations, reduce the concentration of free carriers in the polymer electrolyte.
There is no direct relationship between the dielectric constant of a plasticizer and its ability to associate. For example, although EC and PC have a higher dielectric constant than DMF and DMSO, they are easier to form in the DMF (or DMSO)/PAN/LiClO4 system than in the EC (or PC)/PAN/LiClO4 system. Li+-PAN complex. Although compared with PC or EC, DMF has stronger competitive ability to dissociate Li+-PAN complex, but its dissociation ability to LiClO4 is weaker, and in DMF/PAN/LiClO4 than in EC (PC) /PAN/LiClO4 is easier to form a "Li+-plasticizer molecule" association.
Under normal circumstances, a single-component plasticizer cannot meet all the above requirements, so it is necessary to use a multi-component plasticizer to prepare high ionic conductivity, electrochemical compatibility with the electrode, thermodynamic stability and high enough The mechanical strength of the polymer electrolyte.
Through the above studies on the interaction between the components in PAN-based polymer electrolytes using Raman spectroscopy, infrared spectroscopy, fluorescence spectroscopy, X-ray photoelectron spectroscopy and 7Li nuclear magnetic resonance spectroscopy, the following basic conclusions can be obtained.

X-ray diraction patterns of the PAN-based polymer electrolytes (plasticizer content: 400%)
Lithium ions mainly interact with the plasticizer through the oxygen atoms on the S=O or C=O group of the plasticizer. Increasing the concentration of LiClO4 will not only affect the structure of the plasticizer molecule, but also disturb the symmetry of the ClO4-group, forming ion associations such as solvent-separated ion pairs, contact ion pairs, and ions formed by multiple ions group. The generation of these ion associations will reduce the concentration of carriers. The association number of lithium ions in the EC/LiClO4 solution is about 6.
There is a strong interaction between the plasticizer and the polymer. For example, the interaction between polymer PAN and different plasticizers has different characteristics. The interaction between EC and PAN mainly occurs through the mutual repulsion of dipoles, and for the interaction between DMSO and PAN, the linear diploid produced by the self-association of DMSO molecules must be broken first before PAN and DMSO can occur. The S=O bond interaction of the monomers.
In polymer electrolytes where EC or PC is a plasticizer, the interaction between lithium ions and PAN occurs through lithium ions and the N atoms on the cyano group on the PAN. However, similar interactions were not observed in gel polymer electrolyte systems using DMF or DMSO as plasticizers. This is due to the strong competition between plasticizers and polymers in the association with lithium ions; XPS studies have shown that in PAN-based electrolytes that do not contain plasticizers, the association number of lithium ions is 4 .
Combining the results of Raman spectroscopy, infrared spectroscopy and 7Li NMR, three different states can be identified in polymer electrolytes containing plasticizers: fast-moving lithium ions in the gel state, which is the highest ion conductivity of polymer electrolytes. The important phase corresponds to the sharp peak in NMR; the slow-moving lithium ion in the solid phase has a small contribution to the relative ion conductivity, which corresponds to the broad Lorentz peak in NMR; the chemical shift corresponds to lithium The association formed by the interaction of ions and plasticizers is a common phenomenon in polymer electrolytes containing plasticizers.
Taking into account the competitive effect of plasticizers and polymers in the association with ions and the difference in the movement of different carriers to the gel state and solid phase, when selecting plasticizers for PAN-based polymer electrolytes, An important factor to consider is the competitiveness of plasticizers relative to polymers in association with lithium ions. In this way, more lithium ions can move in the gel state instead of in the solid phase, or form various types of ion pairs or ion groups with the plasticizer, because this will reduce the ionic conductivity of the polymer electrolyte .
















