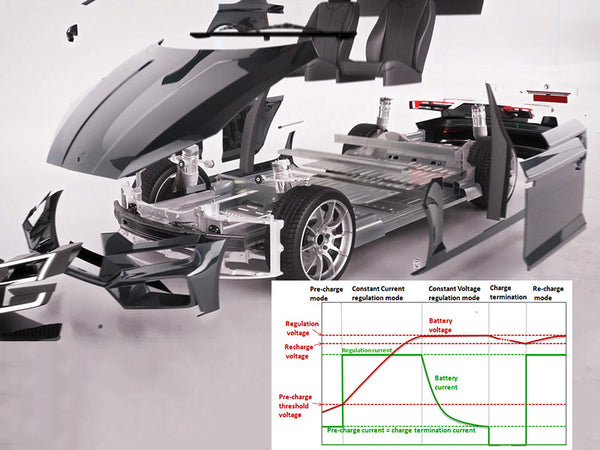
main content:
Lithium is the lightest metal element with an atomic weight of only 6.49 and a density of only 0.534g/cm3. It is also one of the most active metal elements in chemical properties. In the early days, lithium was mainly used in the military industry, and lithium was used to produce tritium, which was an important raw material for the atomic energy industry. Lithium can be used as a battery with high voltage and high specific energy. Lithium metal burns when exposed to air or water. At present, metal lithium is not used in lithium batteries, because lithium metal as the negative electrode will generate dendrites and cause internal short circuits when charging. Lithium alloys or lithium carbides are generally used to replace metal lithium negative electrodes.
Lithium-ion batteries are mainly composed of positive electrodes, negative electrodes, separators and electrolytes. The active materials of the positive electrode materials include lithium cobalt oxide, lithium manganate and lithium iron phosphate, etc. The current collector uses electrolytic aluminum foil with a thickness of 10~20μm; the active material of the negative electrode material is graphite, or carbon with a similar graphite structure, and the conductive current collector uses an electrolytic copper foil with a thickness of 7~15μm; the electrolyte solution is a carbonate-based solvent in which lithium hexafluorophosphate is dissolved, while the lithium polymer battery uses a gel-like electrolyte. The working principle of the lithium-ion battery in the charging/discharging process is described below by taking the lithium manganate battery as an example.
1. The chemical change and ion movement of positive and negative electrode materials during charging/discharging of lithium-ion batteries
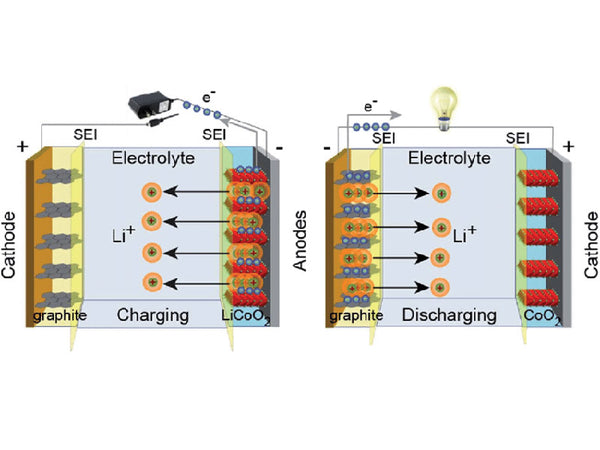
(1) Li migration goes through three stages during charging of Li-ion batteries. First, lithium ions are generated on the positive electrode of the battery, and the generated lithium ions move to the negative electrode through the electrolyte and the diaphragm. The carbon material of the negative electrode has a layered structure with many micropores. The lithium ions reaching the negative electrode are embedded in the micropores of the carbon layer. The more lithium ions are embedded, the higher the voltage difference between the two electrodes of the battery. As the charging process progresses, the negative electrode gradually enters a lithium-rich state, and the positive electrode becomes a lithium-poor state.
During this process, the compensation charge of the electrons is supplied from the external circuit to the negative electrode to maintain the charge balance.
(2) When the battery is discharged, the lithium ions embedded in the carbon layer of the negative electrode come out and return to the positive electrode through movement. The more lithium ions that go back to the positive electrode, the smaller the voltage difference between the two electrodes.
It is not difficult to see that during the charging and discharging process of a lithium ion battery, the lithium ions are in a state of motion from the positive electrode to the negative electrode and then to the positive electrode. If we compare a lithium-ion battery to a rocking chair, the two ends of the rocking chair are the two poles of the battery, and the lithium ions move back and forth at these ends, so it is also called a rocking chair battery.
2. Cathode materials for lithium-ion batteries
The cathode material of the lithium ion battery is made of an embedded compound that can easily insert and extract lithium ions and maintain a stable structure at the same time. Currently, the embedded compounds used as electrode materials are transition metal oxides. During the charge-discharge cycle, lithium ions undergo repeated intercalation and deintercalation reactions on electrodes made of metal oxides. Therefore, the arrangement of oxygen in the crystal structure of metal oxides and its stability are an important indicator of electrode materials.
1) Layered compounds
Lithium cobaltate (LCoO2) and lithium nickelate (LiNO2) are more maturely studied in layered cathode materials. ) lithium cobalt oxide. Lithium cobalt oxide (LCoO2) is the earliest cathode material used in commercial secondary lithium-ion batteries. The traditional solid-phase preparation method of LCoO2 is to use LiCO or a mixture of LOH and CoO to be fired at a high temperature of 900C. Although this preparation method is relatively simple, it is difficult to prepare ideal powders with high purity, small average particle size and narrow particle size distribution. During the charging and discharging process, LCoO2 undergoes a reversible phase transition from trigonal to monoclinic, but this change is only accompanied by a small change in unit cell parameters, so LCoO2 material has good reversibility and cyclic charge-discharge performance. Although LCoO2 has the advantages of high discharge voltage, stable performance, and easy synthesis, it is easy to cause environmental pollution due to the scarcity of cobalt resources, high price, and toxicity. Therefore, lithium-ion batteries using lithium cobalt oxide materials are currently mainly used in small and medium-capacity consumer electronic products such as mobile phones and notebooks.
(2) Lithium nickelate. The properties of nickel and cobalt are very similar, but the price is much lower than that of cobalt, and the pollution to the environment is also less. The most commonly used preparation method of lithium nickelate (LNiO2) is also a high-temperature solid-phase method, that is, a mixture of lithium salt and nickel salt is formed by solid-phase reaction at a high temperature of 700C~850C. LiNO2 has many advantages as a cathode material for lithium-ion batteries, but there are still shortcomings. The main reason is that the cubic LiNO2 is easily generated when the trigonal LiNO2 is prepared. Especially when the reaction temperature is higher than 900C, LiNO2 will be completely transformed from the trigonal system to the cubic system and in the non-aqueous electrolyte solution, the cubic LINIO2 has no electrochemical activity. This shortcoming can be solved by improving the preparation method of LNO2, such as reducing the reaction temperature by soft chemical synthesis method to suppress the formation of cubic LiNO2. At the same time, the doping method (commonly used doping elements are Ti, A, Co, Ca, etc.) can be used to suppress the phase transition during the charging and discharging process, so as to further improve the thermal stability and electrochemical performance of LiNO2.
2) Spinel type structure
Lithium manganate (LiMn2O4) is a typical representative of spinel-type lithium intercalation compounds. Manganese (Mn) is rich in content, cheap and far less toxic than transition metals Co and N. The theoretical discharge capacity of lithium manganate battery is 148mAh/g, and the actual discharge capacity is 110~120mAh/g. The commonly used preparation method of spinel LiMn2O4 is melt impregnation. The method firstly mixes lithium salt and manganese salt uniformly, then heats to the melting point of lithium salt, and utilizes the micropore capillary action of manganese dioxide (MnO2) to fully penetrate the molten lithium salt into the micropores of MnO. In this way, the contact area between the reactants is greatly increased, the uniformity of the product is improved, and the reaction rate of the solid-phase reaction is accelerated. The main disadvantage of LiMn2O4 material is that the cycle capacity of the electrode is easy to decay rapidly, and the reasons for the decrease of cycle capacity mainly include the following aspects
①The octahedral void of LiMn2O4 crystal changes to produce tetragonal distortion. During the charging and discharging process, the electrode surface is easy to form a tetragonal LiMn2O4 crystal with poor stability;
② Manganese in LiMn2O4 is easily dissolved in the electrolyte and causes loss;
③ Electrode polarization causes internal resistance to increase, etc.
In view of the above problems, the spinel structure of LiMn2O4 is stabilized by doping metal ions (such as Cr, Fe, Zn, Mg, etc.), which is one of the most effective methods to solve the cycle capacity decay of lithium manganate batteries.
At present, lithium manganate batteries have been widely used in electric vehicles in demonstration operations. The pure electric buses running during the 2008 Beijing Olympic Games and some electric buses at the 2010 Shanghai World Expo used a single 90Ah lithium manganate battery. This type of lithium-ion battery is also used in the power batteries of the Lea pure electric vehicle launched by Nissan and the iMEV pure electric vehicle launched by Mitsubishi.
3) Olivine type structure
Lithium iron phosphate (LiFePO4) exists in the form of lithium iron phosphate ore in nature, and its crystal belongs to the olivine-type structure of LiFePO4 material. The actual maximum discharge capacity is as high as 164mAh/g, which is very close to its theoretical capacity, and the working voltage is 3.2V. In addition, due to the strong covalent bonds in LiFePO4, it can maintain a high degree of crystal structure stability during charging and discharging, so it has higher safety performance and longer cycle life than other cathode materials. In addition, LiFePO4 has the advantages of wide source of raw materials, low price, no environmental pollution, and high specific capacity, making it one of the hotspots of research in various countries at this stage.
The commonly used synthesis methods of LiFePO4 cathode materials include high temperature solid-phase method and hydrothermal method. The high-temperature solid-phase method is simple and easy to realize industrialization, but the particle size of the product is not easy to control, the shape is irregular, and inert gas protection is required during the synthesis process. The hydrothermal method can directly synthesize LiFePO4 under hydrothermal conditions. Due to the low solubility of oxygen in the hydrothermal system, the hydrothermal synthesis no longer requires the protection of inert gas, and the particle size and morphology of the product are easy to control. At present, the main disadvantage of LiFePO4 cathode material is low electrical conductivity. The effective improvement methods for this problem mainly include surface coating carbon film method and doping method.
At present, the large-scale lithium-ion power battery production plants (such as Hangzhou Wanxiang, Tianjin Lishen, etc.) built in China all take the industrialization of lithium iron phosphate batteries as the main goal. Lithium iron phosphate batteries have also become one of the mainstream products among the electric vehicles that are installed in domestic demonstrations.
3. Anode materials for lithium-ion batteries
Anode material is one of the key factors that determine the overall performance of lithium-ion batteries. High specific capacity, low capacity decay rate, and good safety performance are the basic requirements to be used as negative electrode materials.
1) Carbon material
Carbon materials are currently the most widely used anode materials in commercial lithium-ion batteries. Carbon anode materials include graphite and amorphous carbon, of which graphite is further divided into natural graphite, artificial graphite and graphitized carbon; amorphous carbon can be divided into hard carbon and soft carbon. Graphite is one of the earliest and most studied carbon materials for lithium-ion batteries, which has a complete layered crystal structure. In the lamellar structure of graphite crystal, carbon atoms are combined into a hexagonal network plane by sp2 hybridization. The layered structure of graphite is conducive to the deintercalation of lithium ions to form a lithium-graphite intercalation compound. The theoretical maximum discharge capacity is 372mAh/g, and the charge/discharge efficiency is usually above 90%. The deintercalation reaction of lithium in graphite mainly occurs between 0 and 0.25 V (relative to Li/Li+), indicating that graphite has a good charge/discharge voltage platform, and has a good match with the cathode material that provides the lithium source. The formed battery has a high average output voltage and is a anode material of the lithium ion battery with better performance.
2) Oxide anode material
Oxides are another negative electrode material system currently being studied, mainly including metal oxides, metal-based composite oxides and other oxides. Although the first two have high theoretical specific capacity, the huge capacity loss caused by the replacement of metal element from oxide consumes a large amount of lithium, which offsets the advantage of high capacity;
LixMoO2, LiWO2 and other oxide anode materials have good cycle performance, but due to their low specific capacity, no in-depth research has been carried out so far. The molecule of Li4Ti5O12 has a spinel structure, the charge/discharge curve is flat, and the discharge capacity is 150mAh, which has very good resistance to overcharge and overdischarge. The crystal structure hardly changes during charge/discharge (zero-strain material), the cycle life is long, and the charge/discharge efficiency is close to 100%. It is an ideal anode material with great development potential. It is currently used in energy storage lithium-ion batteries.
3) Metal and alloy anode materials
Metal lithium is the first negative electrode material, with a theoretical specific capacity of 3860mAh/g, an atomic weight of 6.94, and an electrochemical reduction potential of -3.045V. In the mid-1970s, lithium metal began to be used in commercial batteries. However, due to the formation of dendrites on the surface of the negative electrode during charging, the battery is short-circuited, so people began to look for a negative electrode material that can replace metallic lithium. Alloy anode material is a new type of anode material system that has been researched more at present. Metal lithium can form intermetallic compounds with many metals such as aluminum (Al), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), arsenic (As), antimony (Sb), bismuth (B), silver (Ag), gold (Au), and zinc (Zn) at room temperature, so metals that form alloys with lithium can theoretically be used as negative electrodes for lithium-ion batteries. The biggest advantage of metal alloys is that they can form lithium alloys with a high lithium content and have a high specific capacity. At the same time, alloy materials are considered to be a kind of negative electrode material with great development potential due to their advantages such as good processing performance and good electrical conductivity. Current research focuses on zinc (Sn)-based, silicon (Si)-based, antimony (Sb)-based and aluminum (Al)-based alloy materials.
4) The difference between energy-type lithium-ion batteries and power-type lithium-ion batteries
In the process of lithium ion migration, the internal resistance of the chemical structure of the electrolyte, separator, current collector and other parts that constitute the battery body and between the components is called internal resistance of the battery. The difference in the chemical reaction speed of the two poles of the battery and the change of ion concentration near the two poles caused by the movement of ions and charges will cause the electrode potential of the battery to deviate from the equilibrium potential. This phenomenon is called the polarization of the battery. Internal resistance and polarization limit battery performance to some extent. In the production process of power-type lithium-ion batteries for electric vehicles, considering the demand for high-rate current charge/discharge, compared with energy-type batteries of the same capacity, the 4) The difference between energy-type lithium-ion batteries and power-type lithium-ion batteries
In the process of lithium ion migration, the internal resistance of the chemical structure of the electrolyte, separator, current collector and other parts that constitute the battery body and between the components is called internal resistance of the battery. The difference in the chemical reaction speed of the two poles of the battery and the change of ion concentration near the two poles caused by the movement of ions and charges will cause the electrode potential of the battery to deviate from the equilibrium potential. This phenomenon is called the polarization of the battery. Internal resistance and polarization limit battery performance to some extent. The power-type lithium-ion battery for electric vehicles takes into account the demand for high-rate current charge/discharge during the production process. Compared with the energy-type battery of the same capacity, the area of the positive and negative plates is much larger, and the diaphragm is relatively thinner. This makes the impedance between the two poles much smaller. Generally, the DC internal resistance of a 10Ah energy battery is about 10m, while the internal resistance of a power battery is only 12m.
Similarly, the polarization impedance of the power type battery is much smaller than that of the energy type, and the recovery time of the polarization voltage is often within 5s. It is precisely due to the particularity of materials and structures that power lithium-ion power batteries have the following characteristics
(1) High power density. The power density of power lithium-ion batteries is as high as 2000~2500W/kg, but the energy density is low, about 100Wh/kg
(2) Small internal resistance and good rate characteristic. The internal resistance of the battery has a great influence on the power performance of the battery. The smaller the internal resistance of the battery with the same capacity, the smaller the voltage drop when the battery is discharged, and the better the power performance. The advantages of high rate characteristics of power-type lithium-ion batteries are very obvious. Figure 1 shows the discharge curves of power-type lithium-ion batteries at different rate currents. It can be seen from Figure 1 that under normal temperature conditions, the power lithium-ion battery can discharge more than 98% of its rated capacity under constant current discharge at a discharge rate of 1C~5C, and can still discharge 95% of its rated capacity at a discharge rate of 10C.
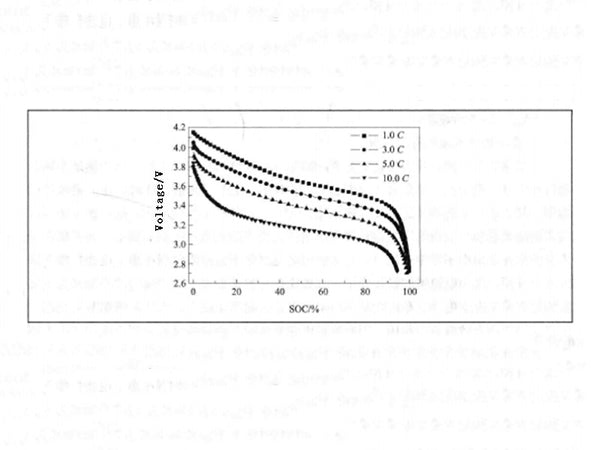
Figure 1 - Discharge curves of power lithium-ion batteries at different rate currents
(3) The polarization recovery time is short. Compared with energy-type lithium-ion power batteries, the polarization recovery time of power-type lithium-ion batteries is very short. The polarization recovery time of general energy-type batteries is about 30s or longer, while the recovery time of power-type lithium-ion batteries is often within 5s.
(4) Long cycle life. In the normal temperature environment, the cycle life test of the power lithium-ion battery for hybrid electric vehicle (HEV) at 1C discharge rate is carried out. After 1600 cycles, the actual capacity of the battery is still more than 80% of the rated capacity, indicating that the cycle characteristics of the power lithium-ion battery are very good. Power-type lithium-ion batteries can meet the requirements of HEV for long-term reliable operation of energy storage devices.