Main content:
Every portable device in the world has some kind of battery or power pack that keeps it functional while in-use. Lipo-battery, for example, is used as built-in panels for mobile phones. These batteries have become so common that we forget one very vital question, how do batteries work? Weirdly, batteries make it possible for our devices to be functional without the need for a cable constantly connected to a power outlet but yet the basic principles of a batter aren’t common knowledge. Well, let’s change that, shall we? In this article, we’re going to break down the most basic principles, components, and concepts that make batteries work.
Before we dive into how do batteries work, let's understand what a battery is. A battery, of course, is a kind of energy conversion and storage device, which transforms the chemical energy or physical energy into electric energy through the reaction.Depending on how much energy can be converted, batteries can be divided into chemical and physical batteries.
1.How do battery systems work?

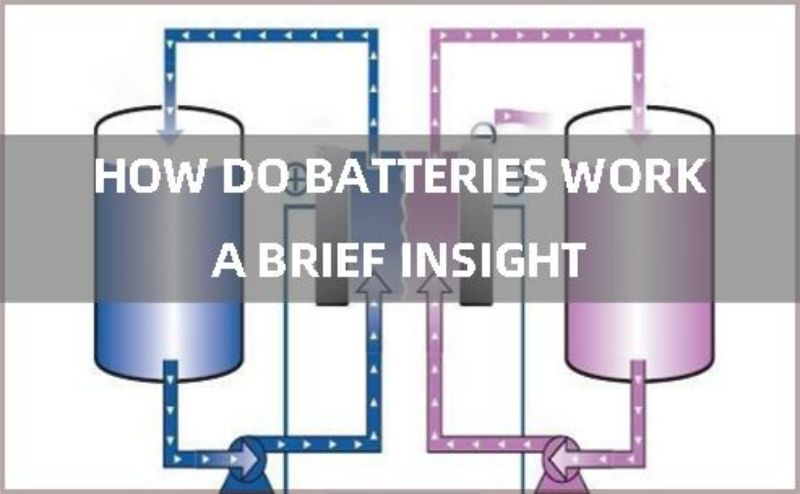
A battery system is comprised of the basic materials that make up the device and are responsible for creating the electrical energy that powers other devices. This system is made up of 3 basic components which are the electrolyte, anode and cathode, and for lithium-ion batteries, an separator is also needed to isolate the positive and negative electrodes.If there is no diaphragm between the positive and negative electrodes, then the positive and negative electrodes will produce a short circuit directly inside the battery, and the battery will not work properly. How do batteries work is by using electrochemical reactions with electrodes in an electrolyte to generate charged ions which circulate the system to supply energy on demand. Let’s break this down a bit.
A single battery cell consists of 2 metal electrodes, the negatively charged anode, and the positively charged cathode. How do batteries work is by generating electrons when the battery is connected to an external power source, the anode then reacts with the electrolyte to generate and release these electrons into the system. The cathode on the other hand becomes positively charged at the same time, pulling these electrons to itself and storing them to be released when the battery is in use. This chemical reaction is reversed during discharge and the electrons transfer and return from the cathode to the anode until they are depleted and the cycle starts again on the next recharge. The electrode reaction must be reversible to ensure the normal process of mass transfer and electricity transmission in the reverse direction.
2.What is chemical reaction of the battery?
We’ve established how do batteries work by recycling charged particles in the system but how do these particles get created and how do they move inside a stationary battery cell? This is all made possible by a chemical reaction called a reduction-oxidation reaction or “redox” reaction for short. This reaction works by an electron transfer through the reducing agent on the negative electrode, electrons are transported to the positive electrode through the external circuit, the oxidant on the positive electrode, so as to complete the electron transfer between the reducing agent and the oxidant.The directional movement of ions in solution between the two poles and the directional movement of electrons in the external wire constitute a closed loop, which makes the reaction of the two electrodes continue, produces an orderly electron transfer process, produces electric current, and realizes the conversion of chemical energy to electric energy and vice versa.
How do batteries work and generates electricity when the battery is charging, the anode reacts within the electrolyte generating electrons and the cathode undergoes a reaction at the same time making it positively charged and ready to receive elections. The generated electrons at the anode accumulate to a point when they move into the electrolyte medium and are pulled to the cathode. This is the power generating concept of a battery and is a standard redox reaction where Reduction occurs at anode that losses electrons while Oxidation occurs at the cathode which gains electrons.
3.How do batteries create electricity?

How do batteries work to create electricity is another interesting concept of a battery system and it also works on the principles of the battery components. To filly grasp how a battery creates electricity we must first understand that electricity is a kind of energy that is generated by the to and fro movement of electrically charged particles in a circuit, closed or open. In the case of a battery, the circuit is a closed medium with two metals of different materials submerged in an electrolyte. The electrolyte is a vital component of electricity generation due to the fact that positive and negative ions are created when certain compounds are mixed in water. This mixture makes up the electrolyte which are basically salts, bases and acids of all kinds.
Although the electrolyte is vital to the electricity generation, it can’t do that alone and this is where the reaction with the electrodes(anode and cathode) come in. So how do batteries work with electrolytes and electrodes to create electricity? The ions in the electrolyte and the different electrode metals at both ends electrochemically react to form the potential difference resulting in the transfer of electrons.The external circuit electronics of the battery move from the low potential to the high potential to form a current from the positive electrode to the negative electrode, and the battery circuit moves from the anode to the cathode to form the cathode to the anode current, forming a closed circuit to provide electricity..
4.What is the basic principle of a battery?

It is important to note that just like every other device, a battery operates on certain basic principles that apply to every battery no matter the size, capacity or brand. How do batteries work based on these principles is what determines how the battery generates, stores, releases and depletes its energy. One key principle governing battery operations is the potential charge difference between two electrodes submerged in an electrolyte medium. This charge difference is what controls the movement of generated electrons within the battery.
How do batteries work following these principles can be simplified into a few steps; When the battery is charged, the battery is connected to an external power source and the electrolytic pool passively performs the redox reaction under an applied power source, the current flows from the positive electrode of the power source through the external circuit to the negative electrode.
In the electrolyte, the positive and negative ions migrate to the poles respectively, and the current flows from the negative electrode to the anode, which is called the discharge of the battery.When discharging, there is chemistry on both electrodes of the battery. The anode losses electrons due to its low electron affinity potential and the cathode accept these electrons due to its high electron affinity potential . When discharging, there are chemical reactions on both electrodes of the battery, the electrical potential difference between both electrodes is then utilized as electricity until the circuit is disconnected or a chemical reaction substance is exhausted, and the cycle starts again on the next charge.
5.Why do batteries run out of power?

How do batteries work on certain principles has been established but how and why does the battery run out of power? Why doesn’t the battery continuously generate electricity infinitely? Well, this question is based on the battery capacity in question. The electrolyte and electrodes within the battery can only generate electrons on a limited base due to the limited capacity of the entire process. After the battery is unplugged from battery charger, the electrodes slowly stop generating electrons with the electron transfer between electrodes used as a source of energy for other devices. How do batteries work is that with no external charge to generate electricity, the power eventually becomes used up and the battery runs out of power.
6.How does a battery become bad?
How do batteries work to generate electricity is an efficient yet imperfect process and these imperfections in the process eventually lead to the battery dying out. The process whereby electrons are generated and transmitted within the battery is never as perfect as the first time the battery is used. With each passing charge cycle, the electrodes become more and more corroded due to the chemical reactions. This corrosion continuously reduces the ability of the electrode to generate and transmit electrons effectively reducing the battery's performance over time. Eventually, the battery's efficiency reduces to a percentage whereby the electrodes are unable to react, meaning the end of life for the battery. Rechargeable batteries such as lithium batteries are designed to last a lot longer than disposable batteries but the same principle applies, only on a much smaller and reduced scale for rechargeable batteries.
7.How does a battery hold a charge?
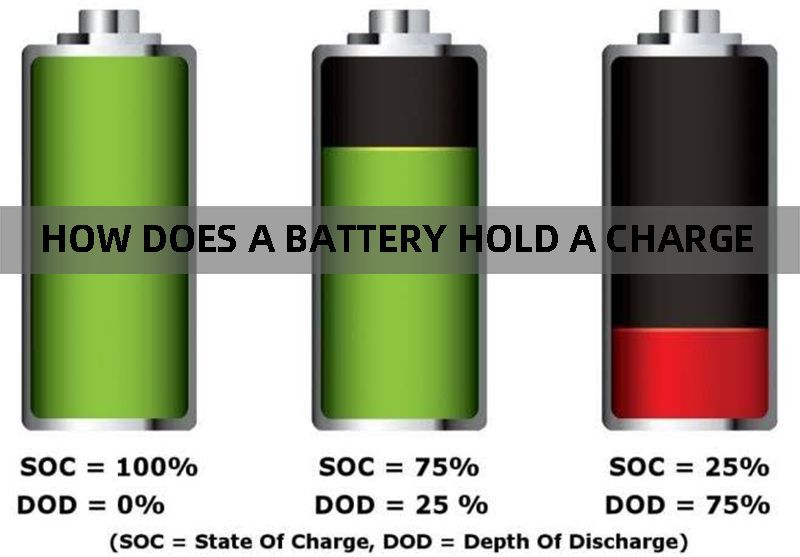
How do batteries work to hold a charge is dependent on the size and battery capacity as well as the battery’s rate of self-discharge. Each battery has a finite number of electrons it can generate and supply and this remains relatively constant as long as the battery is not in use. But even when a battery is not in use, the movement of electrons within the cell is not halted and although the battery might be idle there is still a small amount of charge being lost. This loss of charge is known as self-discharge and varies from battery to battery. A battery with a high rate of self-discharge will lose most of its charge when kept idle, such as nickel-metal hydride batteries, at a self-discharge rate of 30% per month. And vice versa for batteries with a low rate of self-discharge, the self-discharge rate of lithium ion battery is as low as only 3% per month.
















