
Main content:
When it comes to the performance and stability of lithium-ion batteries, electrolyte has always taken center stage. At present, the battery industry has carried out continuous and in-depth research on new lithium salts and solvents, and proposed many methods to improve battery performance and safety, but additives can make up for the shortcomings of electrolyte in some aspects, especially on the surface of positive and negative electrodes. The formation of protective film, has achieved many results. The electrolyte needs to be adapted to the characteristics of the battery system, so the design and research of the electrolyte formulation must be carried out around different battery systems.
1. Conventional electrolyte system
At present, the conventional electrolyte system generally includes organic solvents and lithium salts. EC, DMC, EMC, DEC, and PC are several common organic solvents, and the lithium salt is LiPF6. Studies have shown that EMC and H2O in the electrolyte reduce the thermal stability of IM LiPF6 electrolyte. Among them, EMC is decomposed into DEC and DMC, and DEC and DMC undergo a series of complex organic chemical reactions with the decomposition product PF5 of LiPF6, releasing a large amount of heat and gas, which shows that EMC is used in batteries under high temperature conditions, or for When the battery thermal safety requirements are high, the EMC content in the electrolyte needs to be reduced as much as possible.
At the same time, the influence of water on the thermal stability of the electrolyte was studied, and it was found that the exothermic peak of the electrolyte with a water mass fraction of 5.85×10-3 was at 257℃, and the reaction initiation temperature was 240℃; The exothermic peak of the electrolyte with a fraction of 8 × 10-6 is at 272℃, and the reaction onset temperature is 255℃. The DSC curves of pure LiPF5 and 1mol/L LiPF6 EC-DMC-EMC electrolyte can be seen, the DSC curve shows the first weak endothermic peak at about 195℃, which is LiPF. The melting peak of LiPF6 is reversible; the second endothermic peak appears from around 250℃, indicating that LiPF6 begins to thermally decompose from 250℃. Visible, pure LiPF. Thermally stable up to 250℃. It can be seen that the thermal stability of the electrolyte itself is simultaneously affected by the thermal stability of protic solvents such as water and solvent molecules. The thermal stability of the organic electrolyte of a conventional lithium-ion battery is not bad. The key is that in a real battery, the electrolyte interacts with the positive and negative electrodes in the charged and discharged state, which is the fundamental factor affecting the safety of lithium-ion batteries.

2. Chemical reaction between electrolyte and electrode in lithium ion battery
Lithium-ion battery cathode materials in the charged state, such as LixCoO2, LixNiO2, LixMn2O4, are unstable and decompose, releasing oxygen at high temperatures. The released oxygen reacts with the organic solvent in the electrolyte to generate heat. Under the action of a certain voltage, the solvent and the electrolyte itself may also react, releasing a lot of heat, resulting in safety problems.
Due to the low melting point of the electrolyte, it is difficult to measure its thermal stability. The researchers studied the thermal stability of some lithium-ion battery mixed solvent electrolyte in sealed containers by differential thermal scanning calorimeter. influences. The exothermic peak of the LiPF6 electrolyte containing DEC appears at 255℃, which is 15–20℃ lower than that of the electrolyte containing DMC, which has higher reactivity than DEC. Due to the destruction of the solid electrolyte, the exothermic reaction between 1M LiPF6/EC+DEC, IM LiPF6/EC+DMC and 1M LiPF6/PC+DMC with metallic lithium starts at the melting point of metallic lithium, which is about 180℃, however The self-heating reaction of 1M LiPF6/PC+DEC occurred before. The temperature of the self-exothermic reaction started at 140℃. When water was added to the electrolyte solution containing metallic lithium, the onset temperature of the above exothermic reaction was less than 130℃, which was probably due to the collapse of the SEI film structure caused by HF, which is the reaction product of LiPF6 and water.
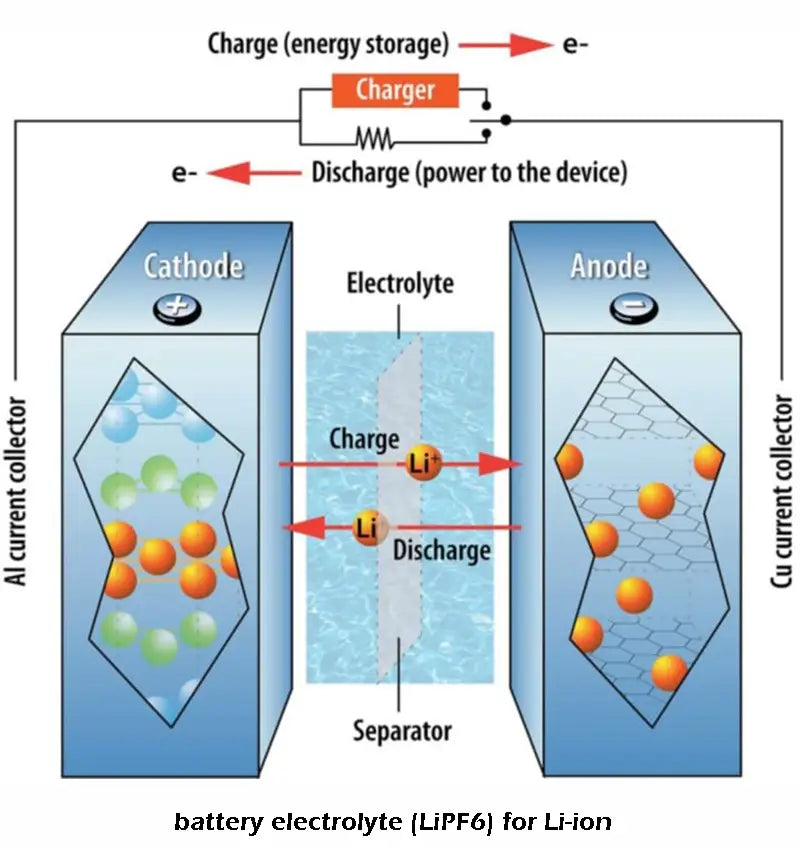
Interfacial reaction between electrode and electrolyte: The instability of cathode material LiCoO2 under high temperature conditions in conventional electrolyte greatly limits its application in large-capacity batteries.
The electrochemical performance of cathode materials strongly depends on the surface chemistry in the electrolyte and the formation of surface films. Similar to the negative electrode, the positive electrode material of many Li-ion batteries can be considered as the SEI electrode. There are many reactions between LixMOy and electrolyte containing carbonate solvents and lithium salts, including irreversible acid-base reactions between LixMOy and trace amounts of HF, O2- in transition metal oxides to solvent molecules with electrophilic properties The initiated nucleophilic attack reaction, the polymerization of cyclic alkyl carbonate on the electrode surface to form polycarbonate, the redox reaction with the composition of the electrolyte, and the dissolution reaction of transition metal ions into the electrolyte. The interfacial reactions and compositions involved in the above can be expressed as follows.
The surface of all cathode materials contains LiF, ROCO2Li, ROCO2M, ROLi, MCO3, Li2CO3, MF2 (M=transition metal), polycarbonate; Li[Mn, Ni]O4→λ-MnO2; LiCoO2→Co3O4; LixMnO2 (layered material) → spinel LiMn2O2 on the surface. Doron Aurbach et al believe that the substances that endanger the performance of the positive electrode are mainly acidic electrolyte, and this is the inevitable result of using LiPF6 as the electrolyte electrolyte. When the acidity of the electrolyte is low and the volume ratio of positive active material to electrolyte is large, the LiCoO2 electrode can cycle well even at temperatures above 60℃. When the electrolyte is contaminated with water and the acidity is high, the performance of the LiCoO2 electrode deteriorates significantly.
High temperature electrolyte: Under high temperature conditions, there are obvious redox reactions between the PF6-anion and the solvent, as well as between all electrolyte compositions and the cathode material. Regarding the mechanism of battery capacity deterioration under high temperature conditions, Doron Aurbach believes that PF6- and its product PF, generate HF with solvent molecules, and HF will react with ROLi, ROCO2Li, Li2O and LiOH, the main components in the solid electrolyte membrane on the negative electrode surface. , LiF is formed and deposited on the surface of the negative electrode. The SEI film containing LiF will seriously hinder the migration of lithium ions, and the higher the enrichment degree, the greater the impact. At the same time, the high-impedance material produced will insulate and isolate the graphite particles. With the continuous charging and discharging under high temperature conditions, the electrode interface impedance and the insulation isolation between the active material and the conductive material will continuously lead to the deterioration of the negative electrode performance, and finally lead to the lithium-ion battery capacity too low to fail.
















