- 1.Li4/3Ti5/3O4 structure and lithium insertion characteristics
- 2.Preparation method of Li4/3Ti5/3O4
- 3.Doping modification of Li4/3Ti5/3O4
Li4/3Ti5/3O4 is another lithium-containing negative electrode material, and its structure is spinel type. As a negative electrode material, Li4/3Ti5/3O4 has a stable structure and hardly changes in volume during charging and discharging. Therefore, it has very good cycle performance. At the same time, titanium resources are abundant, clean and environmentally friendly; but Li4/3Ti5/3O4 has a lithium insertion potential High (1.55V), if LiCoO2 is directly used as the positive electrode to compose the battery, it will inevitably reduce the output voltage of the battery, and the 5V positive electrode material is also a problem for the electrolyte requirements, so Li4/3Ti5/3O4 also has a certain degree as a negative electrode material. limit.
1.Li4/3Ti5/3O4 structure and lithium insertion characteristics

Li4/3Ti5/3O4 is a composite oxide composed of metallic lithium and low-potential transition metal titanium, belonging to the AB2X4 series, and its structure can be expressed as Li[Li1/3Ti5/3]O4. Li4/3Ti5/3O4 is a cubic crystal system, the spatial point group is Fd3m, and the unit cell parameter a=0.836nm. Its crystal structure is shown in Figure 1. The complete unit cell contains 8 Li4/3Ti5/3O4 structural units, 32 oxygen ions are arranged according to FCC (face-centered cubic stacking), located at the position of 32e; 3/4 of the total lithium ion position 8a is located at the tetrahedral position, The remaining lithium ions and Ti4+ are located in the 16d octahedral position. The structure of Li4/3Ti5/3O4 provides a three-dimensional diffusion channel for the deintercalation of lithium.
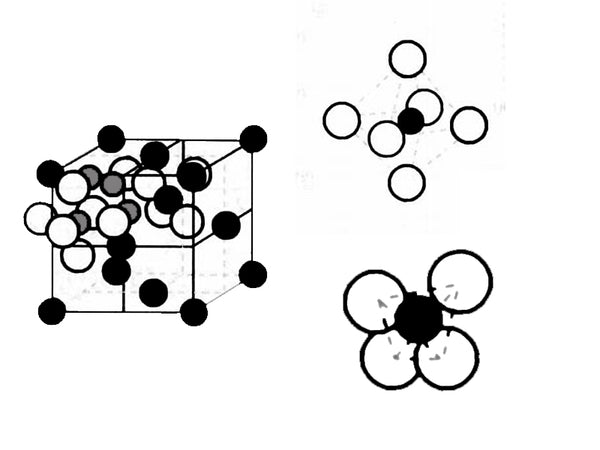
Figure 1 Schematic diagram of the crystal structure of Li4/3Ti5/3O4
During the charging process of Li4/3Ti5/3O4, the intercalated lithium ions occupy the octahedral position (16c). At the same time, the lithium originally in the 8a tetrahedral position also migrates to the octahedral position 16c, forming a new spinel phase Li7/3Ti5/ 3O4. Li4/3Ti5/3O4 can insert at most one lithium during the charging process, so its theoretical capacity is 175mA·h/g. The reaction of Li4/3Ti5/3O4 during the charging and discharging process is as follows:
Li[Li1/3Ti5/3]O4+xLi↔Li1+x[Li1/3Ti5/3]O4
8a 16d 32e 16c 16d 32e
In the formula, the intercalation of Li+ in Li4/3Ti5/3O4 is a two-phase process, and this transformation is highly reversible in kinetics. With the intercalation of lithium, Ti4+ is reduced to Ti3+. Li4/3Ti5/3O4 has the same structure as its charged product Li7/3Ti5/3O4, both of which are cubic spinel structure, except that the unit cell parameter a changes slightly from 0.836nm to 0.837nm, and the change in unit cell volume is very small. It is called "zero strain material". They are different in physical properties, as shown in Figure 2. It is precisely because Li4/3Ti5/3O4 hardly changes its structure before and after charging and discharging, it is used as a negative electrode material for lithium-ion batteries, which has very good cycle performance and can maintain a stable capacity after thousands of cycles.
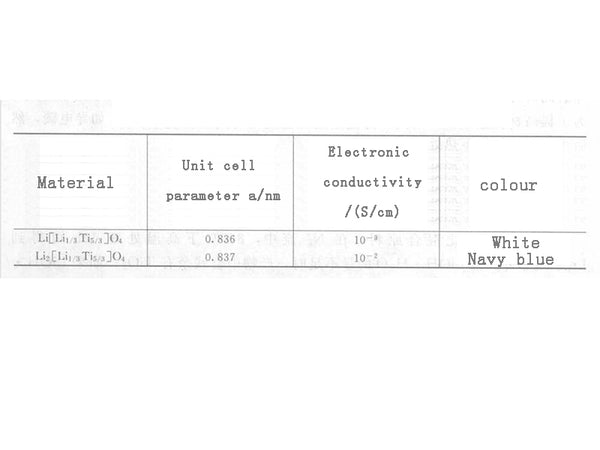
Figure 2 Structural parameters of Li4/3Ti5/3O4 and Li7/3Ti5/3O4
The typical charging and discharging curve of Li4/3Ti5/3O4 is shown in Figure 3. It can be seen from the figure that the lithium insertion voltage of Li4/3Ti5/3O4 is 1.55V, the charging and discharging platform is very flat, and the Coulomb efficiency reaches 100%. , The cycle performance is also very good, the capacity has not been significantly attenuated after 100 cycles. Figure 4 is the cyclic voltammetry curve of Li4/3Ti5/3O4 scanned at a scan rate of 10μV/s in 1mol/L LiClO4/PC electrolyte. The redox peaks are very symmetrical, and the peak potential difference is 60mV, indicating The electrode process of Li4/3Ti5/3O4 is reversible.

Figure 3 Charging and discharging curves of Li4/3Ti5/3O4
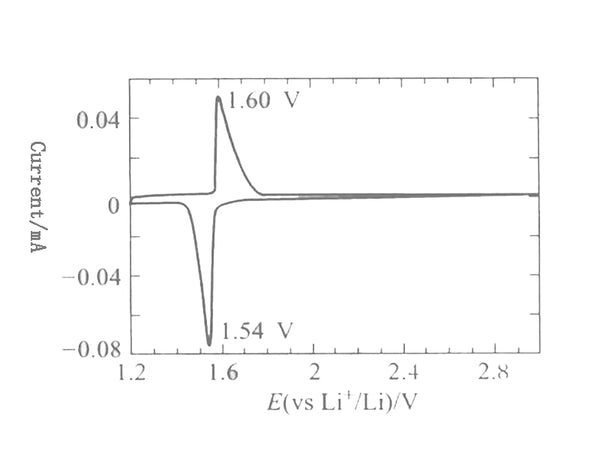
Figure 4 Li4/3Ti5/3O4 cyclic voltammogram(Scan rate 10AV/s)
The insertion voltage of Li4/3Ti5/3O4 is 1.55V, which is higher than that of graphite materials. Therefore, it requires a higher voltage of the positive electrode material that composes the battery. If the positive electrode is 4V LiCoO2 or Li2MnO4, the battery voltage is 2.5V. To form a battery with 5V cathode material LiCoPO4, the battery reaches 3.5V, but the requirements for electrolyte are very strict.
As a negative electrode material, Li4/3Ti5/3O4 has good high-temperature performance. This is because Li4/3Ti5/3O4 has poor conductivity. Increasing the battery's operating temperature can increase the intrinsic conductivity of the material and make the material more effective. Good rate performance. Under 10C charge and discharge, the capacity at 50°C is about 20mA·h/g higher than that at 25°C.
2.Preparation method of Li4/3Ti5/3O4

The preparation methods of Li4/3Ti5/3O4 mainly include solid-phase reaction and sol-gel method. Their advantages and disadvantages are similar to those of other electrode materials: the solid-phase method has simple process, requires higher heat treatment temperature and longer The sintering time is large, the energy consumption is large, and the particle size is large, which is difficult to control; while the sol-gel method can make up for the shortcomings of the solid phase method, but introduces a large number of organic compounds, and the process is also more complicated.
The synthesis process of Li4/3Ti5/3O4 by solid-phase reaction is similar to other metal composite oxides, usually TiO2 is mixed with LiOH·H2O or Li2CO3, and then treated at high temperature (800~1000℃) for 12~24h to obtain the product Li4/3Ti5 /3O4. In order to achieve the purpose of fully and uniformly mixing the raw materials, high-energy ball milling can be used. Ball milling can shorten the reaction time and lower the heat treatment temperature to obtain particles with smaller particle size and narrower distribution. In order to improve the conductivity of the material, a certain amount of inactive conductive agent, such as conductive carbon, can be added to the raw material, and then heat treated in an inert atmosphere.
The raw materials are mixed according to the stoichiometric ratio, and Li4/3Ti5/3O4 can be obtained by high-temperature treatment at 800°C for 12h in a stream of N2. When the amount of LiOH·H2O is insufficient, TiO2 will remain in the product; if the amount of LiOH·H2O is excessive, the product will have monoclinic crystal Li2TiO3. The product is charged and discharged at 0.17mA/cm² under LiNiO2 as the positive electrode. The first discharge capacity is 150mA·h/g, but compared with the natural graphite negative electrode, its capacity decays faster.
After TiO2 and Li2CO3 are mixed by ball milling, they are pre-fired at 600~700℃ for several hours and then ground, and calcined at 900~1000℃ for 1~2h to obtain Li4Ti5O12. Charge and discharge under C/10 rate, the first discharge specific capacity is 170mA·h/g, the maximum calcination temperature of Li4/3Ti5/3O4 is (1015±5) ℃, beyond this temperature, it will decompose into Li2TiO3 and Li2Ti3O7 Mixed phase.
N2 is not a necessary condition for the preparation of Li4/3Ti5/3O4. Some people use Li2CO3 as a lithium source. After heat treatment at 1000℃ for 26h, Li4/3Ti5/3O4 with excellent electrochemical performance is obtained. In the preparation process, the raw material lithium salt is generally excessive 8 %about.
The sol-gel method is a liquid phase synthesis method, which can synthesize nano-level ultrafine powders. Generally, organic precursors are used as raw materials to form sols through hydrolysis or alcoholysis of the raw materials. The sols volatilize solvents and age under certain conditions. Loss of fluidity to obtain gel, and then heat treatment to obtain the final product.
Add an appropriate amount of titanium isopropoxide Ti[OCH(CH3)2]4 to the methanol solution of lithium acetate to obtain a yellow colloid. Stir for 1 hour to obtain a white gel. Dry at 60°C for one day to obtain a dry gel. The dry gel is calcined at 700~800℃ to obtain the product. The Li4/3Ti5/3O4 prepared by this method has a lithium insertion potential of 1.55V and a capacity of 95% (C/60) of the theoretical capacity.
Using n-butyl titanate [Ti(OC4H9)4] and lithium acetate as raw materials, using isopropanol as the solvent, the nano-scale Li4/3Ti5/3O4 was synthesized through a sol-gel route, and the average particle size obtained was 100nm. The product was charged and discharged at 0.3mA/cm², and the first lithium insertion capacity was as high as 272mA·h/g, but the first discharge efficiency was as low as 53.9%, and good cycle performance was maintained thereafter.
3.Doping modification of Li4/3Ti5/3O4

The main disadvantage of Li4/3Ti5/3O4 as a negative electrode material is that its lithium-intercalation potential is relatively high, which makes the output voltage of the battery low; at the same time, the electronic conductivity of Li4/3Ti5/3O4 is low, and the high-current charge and discharge performance is poor. Therefore, the modification of Li4/3Ti5/3O4 is mainly to reduce its lithium insertion potential and increase its electrical conductivity to improve high-current charge and discharge performance.
(1) Modification to reduce lithium intercalation potential
Substituting transition metal elements (such as Fe, Ni, Co, etc.) for Ti4+ in Li4/3Ti5/3O4 can reduce the lithium intercalation potential of lithium titanate to a certain extent without changing the spinel of Li4/3Ti5/3O4 structure. Generally, the oxide is selected for doping, the reactant and the doped oxide are mixed and ball-milled, and then sintered at a high temperature. Most of the doped metal atoms occupy the 16d position, only Fe occupies a small amount of 8a positions, and produce cation vacancies; the doping of nickel and chromium leads to the appearance of 0.6V discharge platform, and increases the capacity by about 40mA·h/g , But there is a significant capacity attenuation. Iron doping forms a solid solution Li1+xFe1-3xTi1+2xO4 (0≤x≤0.33), and the discharge platform is below 1.5V, but with the increase of iron doping, the capacity of the material drops quickly, and the cycle performance can only be maintained at 25 Within times; when the iron atom doping amount is the largest (x=0), the formed LiFeTiPO4 has an initial capacity of up to 230mA·h/g, but the efficiency of the first cycle is very poor, and the second cycle is below 150mA·h/g , And then the capacity decays faster.
The doping modification of Li4/3Ti5/3O4 to reduce the lithium insertion potential does reduce the electrode potential to a certain extent, but it also weakens the advantages of the material in terms of cycle stability to varying degrees. This work It remains to be further studied.
(2) Modification to improve high-current charge and discharge capabilities
There are two main methods, one is to reduce the particle size, shorten the diffusion distance of lithium ions, and increase the diffusion coefficient of lithium ions; the other method is doping, which includes bulk doping of the material with metal ions to form a solid solution, or It is the introduction of conductive agent carbon to improve conductivity.
Nano-scale Li4/3Ti5/3O4 has better capacity performance than ordinary Li4/3Ti5/3O4 at larger currents. Taking the specific capacity of the two at 0.5C discharge as a reference, When discharged at 1C, nano- Li4/3Ti5/3O4 can release 94%, the latter can release 80%; the former can release 75%, and the latter can release 30%. To obtain nano-scale particles, it can be achieved by improving the synthesis method of materials. The liquid phase method can obtain ultrafine particles, even nano-sized particles. Since Ti4+ is easy to form a sol, the sol-gel method is often used to synthesize Li4/3Ti5/3O4.
The intrinsic conductivity of semiconductor materials can be improved by introducing impurity ions. When the valence state of the impurity ion is inconsistent with the valence state of the ion it replaces, it will bring extra charges to the crystal, and these extra charges must pass through other opposite charges. The impurity ions are compensated or offset by creating vacancies, so that the entire crystal remains electrically neutral. When high-valent cations are used to replace low-valent cations, cation vacancies will be generated, and when low-valent ions are substituted for high valences, anion vacancies will be generated. Both cation vacancies and anion vacancies will cause distortion of the crystal lattice, cause defects, and make the crystal The conductivity rises.
Substituting magnesium for lithium can obtain solid solution Li4-xMgxTi5O12 (0.1≤x≤1.00). Since magnesium is a divalent metal and lithium is monovalent, part of the titanium changes from tetravalent to trivalent, and the appearance of mixed valence improves the material Electronic conductivity. When x=1.00, the conductivity of the material can be increased to 10-2S/cm². The substitution of trivalent chromium ions for lithium also has a similar effect.
Carbon doping with Li4-xMgxTi5O12 can improve its electronic conductivity. This doping is different from the previous doping of metal ions. The doping of metal ions introduces vacancies and improves the intrinsic conductivity of the material; while the doping of carbon is To reduce the contact resistance and at the same time play a role in dispersion, this doping is a bulk doping. For Li4/3Ti5/3O4, the doped carbon element can be activated carbon and natural graphite with specific surface area of 80m²/g and 2000m²/g respectively. The particle diameter of the material is about 50nm, and its specific capacity and circulation are excellent.
Except Li4/3Ti5/3O4 can be used as the negative electrode of lithium ion batteries, some other titanium oxides can also reversibly store lithium. For example, Li2MTi6O14 (M=Sr, Ba) is a cubic crystal system with three-dimensional lithium insertion channels. Li2MTi6O14 can embed a maximum of 4 lithium, there are three platforms at 1.4~0.5V, and its actual capacity is about 140mA·h/g.