Carbon nanomaterials mainly refer to carbon nanotubes, amorphous carbon materials with nanoporous structures, natural graphite, and nano-doping of carbon materials.
Carbon nanotubes

Carbon nanotubes (CNTs,) were discovered in 1991 by S.lijima, an electron microscope expert at NEC Corporation, Japan. He used an argon DC arc to discharge the cathode carbon rods, and found a tubular structure of carbon atom clusters, that is, carbon nanotubes. Carbon nanotubes are multilayer tubulars and particulates with nano-sized carbon, such as Buckytubes and Buckyonlons. It is a hollow tube with a diameter of several nanometers and several tens of nanometers, and a length of several tens of nanometers to 1 μm. The special structure of the tube makes the lithium ion extraction depth small and the process short, which is beneficial to improve the charge and discharge capacity and current density of the lithium ion battery.
(1) The structure and classification of carbon nanotubes. Nanotubes are seamless nanotubes formed by crimping single or multilayer graphite sheets. Each nanotube is a cylindrical surface composed of a hexagonal plane with a carbon atom completely bonded to the surrounding three carbon atoms through sp2 hybridization; the side length of the planar hexagonal unit cell is 0.246nm, and the shortest carbon-carbon bond length is 0.142nm. The interlayer of the multilayer nanotubes is close to the ABAB-type stacking, and the sheet spacing is generally 0.34nm, which is basically the same as the graphite sheet spacing (0.335nm). The top of each single-layer tube is closed by a pentagon or heptagon. Carbon nanotubes are mostly multi-layered tubes, which are closed and bent. This is because pentagons and heptagons are introduced into the hexagon. During the growth process, the hexagonal ring needs to add two carbon atoms to the surrounding nodes. If the carbon supply is reduced, only one carbon atom enters, resulting in the formation of a pentagonal ring, causing positive bending; on the contrary, the high flow rate of carbon atoms is conducive to the formation of a heptagonal ring, where three carbon atoms enter into the bond and cause negative bending. The pentagonal ring and the heptagon as a whole are introduced into the bend of the carbon nanotube to be continuous. From the topological analysis, the pentagon and heptagonal ring should appear in pairs at the bend.
There are many types of carbon nanotubes, which can be divided into single-walled carbon nanotubes and multi-walled nanotubes according to the number of walls (graphite sheets); according to the degree of graphitization, it can be divided into amorphous carbon nanotubes and graphitized carbon nanotubes; according to the helix angle (the smallest angle between the carbon-carbon bond and the plane perpendicular to the cylinder axis), it can be divided into spiral carbon nanotubes and non-helical carbon nanotubes; according to whether the two ends of the nanotubes are closed, it can be divided into open carbon nanotubes and closed carbon nanotubes; according to the configuration, it can be divided into three types: armchair carbon nanotubes, zigzag carbon nanotubes and chiral (spiral) carbon nanotubes, as shown in Figure 1.

Figure 1 - Schematic diagram of the structure of single-walled carbon nanotubes
The wall of single-walled carbon nanotubes is composed of a layer of carbon atoms, which is the limit form of carbon nanotubes, with a diameter of 1~2nm; while multi-walled carbon nanotubes are composed of several to dozens of single-walled carbon nanotubes coaxially. The force between the tubes is the same as that between the graphite layers, which is van der Waals force. Non-helical carbon nanotubes refer to carbon-carbon bonds perpendicular to the cylindrical axis (helix angle θ=0°), and the curling direction at this time [1010]*; or the carbon-carbon bond is parallel to the cylinder axis (helix angle θ=30°), at this time, the curling direction [1120]*, the non-helical carbon nanotube periodic structure appears with a lattice constant. In other cases, the cycle is longer, called helical carbon nanotubes.
Carbon nanotubes have excellent physical and chemical properties. For example, it has a large specific surface area (up to 250m3/g) and molecular size pores, extremely high tensile strength (11~63GPa), good thermodynamic properties, high chemical stability (there is basically no mass loss below 625℃, and the highest mass loss rate at 960℃ is only 2.1%), good hydrogen storage performance, etc. Therefore, many researches have been conducted in various fields such as catalyst carriers, hydrogen storage materials, lithium ion batteries, electric double layer capacitors, and field emission.
(2) Preparation of carbon nanotubes. There are many methods for the preparation of carbon nanotubes. Commonly used are arc discharge method, catalytic pyrolysis method and laser evaporation method (see Figure 2). Other methods include microporous template method, plasma jet decomposition deposition method and diffusion flame method. The preparation of multi-walled carbon nanotubes is relatively mature, and the diameter and orientation of the product can be controlled. However, the output of single-walled carbon nanotubes is only gram-level, and it is difficult to obtain single-walled carbon nanotubes of the desired structure. The following mainly introduces the arc discharge method and the catalytic pyrolysis method.

Figure 2 - Schematic diagram of carbon nanotube preparation equipment
① Arc discharge method. The arc is essentially a gas discharge phenomenon, which makes the gas space between the two electrodes conduct electricity under certain conditions, which is the process of converting electrical energy into heat and light energy. S. lijima, who first discovered carbon nanotubes in 1991, produced multi-walled carbon nanotubes by the arc discharge method. The electric discharge method is an early method of preparing carbon nanotubes, and the carbon nanotubes prepared by the arc method have been commercialized. The preparation device of the arc discharge method is complicated, but the process parameters are easier to control; the product carbon nanotubes have straight morphology and good crystallinity, but the yield is low and the purity is low, making separation and purification difficult. The arc discharge method is divided into graphite arc method and catalytic arc method.
② Catalytic pyrolysis method. Catalytic pyrolysis is a method of preparing carbon nanotubes by decomposing gas (or vapor) containing carbon sources when flowing over the surface of a metal catalyst. Catalytic pyrolysis has the advantages of low cost, large output, and easy control of experimental conditions. It is a method for the experimental large-scale preparation of high-quality multi-walled carbon nanotubes. The catalyst used in this method is generally Fe, Co, Ni and their alloys of group VIII, and sometimes alloys doped with rare earths or other elements and compounds are also used. Carbon sources are divided into gas and liquid. Gases are generally CH4, CO, C2H4, C2H2, etc.; liquids include benzene, xylene, ferrocene, cyclohexane, C10H10, etc.; carrier gas is mostly H2, Ar, N2, etc. Catalytic pyrolysis can be divided into three methods: matrix method, spray method, and flow catalysis method according to the way of catalyst addition or existence.
The type and preparation method of the catalyst used in the catalytic pyrolysis reaction, the carrier, the type, ratio and flow rate of the reaction gas, and the reaction temperature all have an impact on the quantity, quality, inner and outer diameter, and length of the produced carbon nanotubes. In the reaction, the commonly used catalysts are generally Fe, Co, Ni, Cr and other metal elements or their alloys supported on silica gel or molecular sieve or graphite. Carbon nanotubes catalyzed by Fe and Co have a good degree of graphitization, and the catalytic performance of cobalt is better than that of iron. Carbon nanotubes prepared with copper as a catalyst are amorphous carbon. The use of silica gel as a support agent can make the catalyst metal particles better disperse, the prepared carbon tube is finer, and the size distribution is more uniform. The catalyst can be in various forms, it can be a supported type, it can also be a solid solution, a mesh, a pure metal or an alloy. The mixed gas used is generally ethane and nitrogen.
The diameter of carbon nanotubes largely depends on the diameter of the catalyst particles. Therefore, by selecting the type and particle size of the catalyst and controlling the process conditions, carbon nanotubes with higher purity and uniform size distribution can be obtained. The carbon nanotubes prepared by this method have crystal defects, such as bending and deformation, and the degree of graphitization is poor, so certain post-processing is required, such as high temperature annealing treatment can eliminate some defects, straighten the tube and increase the degree of graphitization.
(3) The electrochemical properties of carbon nanotubes. Carbon nanotubes used as anode materials for lithium-ion batteries have the advantages of small embedding depth, short process, multiple embedding positions (gaps and holes in the tube and between layers), and large lithium storage (up to CLi2 level). At the same time, carbon nanotubes have good electrical conductivity, which are beneficial to the charge and discharge performance of carbon nanotubes; however, there are also some disadvantages, such as excessively high irreversible capacity, voltage hysteresis, and unobvious discharge platform. In addition, carbon nanotubes also have an obvious electric double-layer capacitance (35F/g) effect, and the charge transfer rate needs to be further improved.
The reason for the excessively high irreversible capacity of carbon nanotubes may be: there is a strong tendency to accumulate charges in the carbon nanotubes. Due to the electrostatic attraction of the charges, it is difficult for lithium ions to escape once inserted into the inner pores of the carbon nanotubes; the internal entanglement of carbon nanotubes; the formation of SEI film, due to the very large specific surface of the carbon nanotubes, the SEI film continues to form and age during the cycle.
Regarding the capacity loss during battery cycling, it is generally believed that the intercalation/deintercalation of lithium and the co-intercalation of solvent molecules cause the graphite layer of carbon nanotubes to fall off, which is the main reason for the loss of electrode capacity.
The voltage hysteresis of carbon nanotubes is manifested by the insertion of lithium ions at about 0V when charging, but around 1V when discharging, which is similar to the charge and discharge characteristics of graphitized carbon materials. Some people think that there are many defects on the edge of the carbon nanotube lattice, which promote the insertion of lithium ions during carbon reduction, and the extraction of lithium ions during oxidation is accompanied by great resistance and overpotential; it is also believed that this is related to the hydrogen contained in the carbon nanotubes, and others believe that the potential hysteresis is caused by the existence of interstitial carbon atoms.
When carbon nanotubes prepared by chemical vapor deposition are used as the negative electrode active material of lithium-ion batteries, the battery capacity exceeds the theoretical capacity of graphite lithium intercalation compounds by more than one time. Carbon nanotubes with a lower degree of graphitization can have a capacity of 700mA·h. /g, but there is a potential hysteresis of about 1V, and the carbon nanotubes with a higher degree of graphitization have a lower capacity (300mA·h/g), but the potential hysteresis is smaller and the cycle stability is significantly improved.
Certain factors, such as morphology, microstructure, degree of graphitization, impurity atoms, and surface chemical composition, have an impact on the lithium insertion performance of carbon nanotubes. The diameter, wall thickness and other morphological parameters of carbon nanotubes can be adjusted by changing the catalyst preparation process and conditions; under the same current density, its reversible capacity varies from 180 to 560 mA·h/g. Carbon nanotubes with short length, thick tube wall, small tube cavity and irregular surface have high lithium insertion capacity and good reversibility. After the carbon nanotubes are subjected to high temperature annealing heat treatment, their BET surface area and pore volume decrease with the increase of heat treatment temperature, and their irreversible capacity and reversible capacity also decrease accordingly. The potential hysteresis is related to the microstructure of carbon nanotubes and the oxygen-containing groups on the surface. If the microstructure of carbon nanotubes can be effectively controlled and the influence of interstitial carbon atoms and surface groups can be eliminated, the potential hysteresis in the process of lithium insertion can be eliminated (see Figures 3 and 4).
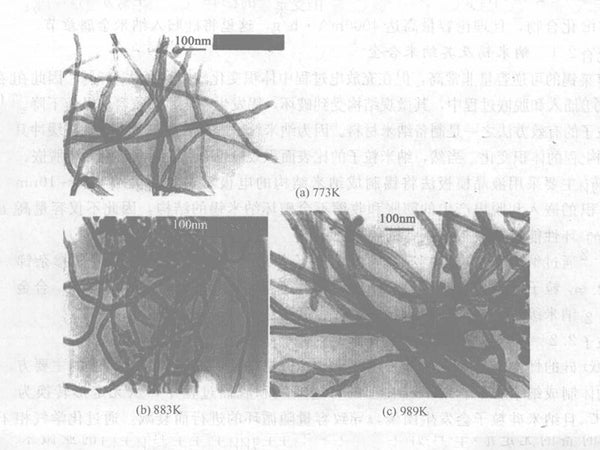
Figure 3 - TEM images of carbon nanotubes treated at different temperatures
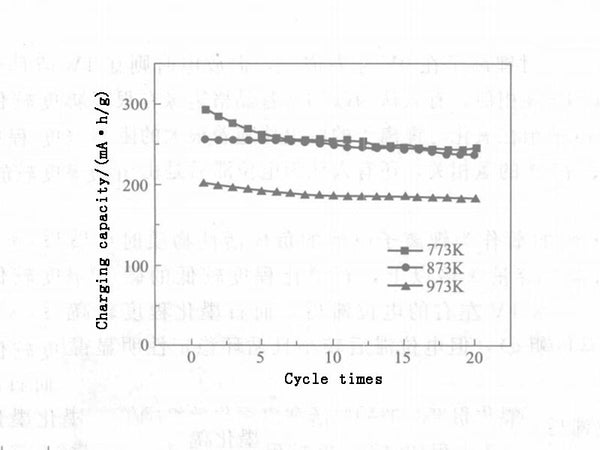
Figure 4 - Cyclic performance of carbon nanotubes at different temperatures
Carbon nano-doped materials

Carbon nano-doped materials refer to doping other atoms in the carbon material structure, and these atoms are present in the carbon structure in nanometer size. The most typical carbon nano-doped material base is the nano-doping of silicon atoms in the carbon material. Since silicon and carbon have similar chemical properties, it can bond closely with the surrounding carbon atoms. At the same time, because carbon atoms are doped with silicon atoms and these silicon atoms are nano-dispersed in the carbon material structure, lithium ions can not only be embedded in the carbon material structure, but also in the gaps of nano-scale silicon atoms.
In theory, each silicon atom can be combined with four lithium atoms, so nano-doping of silicon atoms in carbon materials can increase the insertion position of lithium ions, provide a large number of nanochannels for lithium ions, and increase the lithium insertion capacity of carbon materials. In addition to silicon, dopant atoms of carbon materials include phosphorus, nickel, and lead. There are many types of carbon used as anode materials for lithium-ion batteries, such as graphite, MCMB, carbon fiber, pyrolytic carbon, and so on. These carbon materials can be doped with impurities to change their properties, and there are also many kinds of doped atoms, such as silicon, phosphorus, and fluorine.