
The battery recovery process is essentially a mixture separation process, the most important of which is the process of metal separation and separate extraction and purification. Therefore, the starting point of nickel-cadmium battery recycling technology is the separation and purification technology of nickel, cadmium and cobalt.
There are generally two processes for the recovery of waste nickel-cadmium batteries: fire method and wet method. The most important way to recover cadmium in nickel-cadmium rechargeable batteries is to separate nickel-cadmium. The wet process mainly utilizes the different properties of each component of the battery for recovery in solution; the fire process mainly utilizes the different oxygen potential and vapor pressure of each component in the battery for separation and recovery.
1. Wet recycling process of waste nickel-cadmium battery
The basic process of the waste nickel-cadmium battery wet recovery process: first, completely crush and clean the waste battery; then chemical or biological methods are used to convert the solid state metal into metal ions into the solution; then, various valuable metal ions are separated, and finally products containing metal ions are obtained by precipitation or drying technology.
2. Selective leaching process of waste nickel-cadmium batteries
The main principle of the process is to use the different solubility of each component of the nickel-cadmium battery in the solution, and to achieve the purpose of selective leaching by selecting the solution, adjusting the pH value and the temperature.
(1) Selective leaching of nickel. The main steps are as follows: firstly, the waste battery is broken and cleaned to remove the potassium hydroxide electrolyte therein;the cleaned substances are oxidized and fired at 550~600°C for more than 1 hour, so that the metallic cadmium is converted into cadmium oxide, and the cadmium hydroxide and nickel hydroxide in the battery are decomposed into oxides; then, the calcined oxide is leached in NH4NO3 solution with a concentration of 4 mol/L, cadmium oxide can be dissolved in NH4NO3 solution, while nickel and iron are insoluble in NH4NO3 solution; passing through CO2 causes cadmium ions to form CdCO3 precipitation, so that cadmium is separated from the solution.
The disadvantage of this process is that the resulting CdCO3 product contains 0.14% Ni and 0.12% Co impurities. The leaching rate of cadmium is only 94%. In addition, specialized oxidative roasters are required. There is a large amount of CO2 emission during this operation, and the operation cost is high.
(2) Bioleaching of waste nickel-cadmium batteries. Thiobacillus ferrooxidans is a commonly used bioleaching strain, which can use the energy generated during the oxidation of sulfur to grow and produce a series of sulfur compounds, of which sulfuric acid is its final product. Thiobacillus ferrooxidans has strong resistance to toxic metals nickel and cadmium, which is of great significance for its use as a bioleaching agent for waste nickel-cadmium batteries. When the operating conditions are good, in the reactor where Thiobacillus ferrooxidans exists, the highest concentration of hydrogen ions can reach 80mmol·kg-1·d-1. The operation process is shown in Figure 1.

The reactor consists of two percolators. Add 160 g of sulfur to the first percolator, and the air flow rate is 120L·h-1; then add 20 mL of culture medium containing Thiobacillus ferrooxidans and 180 mL of iron-free culture medium with an original pH of 2.0. When the system temperature was 30℃, the growth rate of Thiobacillus ferrooxidans increased exponentially. The waste nickel-cadmium batteries were put into the second percolator containing the culture solution of Thiobacillus ferrooxidans (pH=1.0) for dissolution. When the pH of the solution reached 2.5, the solution was replaced with fresh medium. After 93 days, the dissolution rates of cadmium, nickel and iron were 100%, 96.5% and 95.0%, respectively. In addition, when the positive and negative electrodes and sulfur of the nickel-cadmium battery were directly added to the culture solution of Thiobacillus ferrooxidans, the dissolution rate was also higher than 90%. This method can be used as the first step in the recycling of waste nickel-cadmium batteries, but the main disadvantage is the long processing cycle.
3. The selective precipitation process of cadmium
The selective precipitation process of cadmium is based on the principle that the solubility products of cadmium carbonate and nickel carbonate are quite different for separation, and Cd and Ni are recovered from the solution containing Cd and Ni respectively.
The main steps of the selective precipitation process of cadmium are as follows: first, the waste battery is dissolved in hot sulfuric acid with pH=4~4.5, so that nickel and cadmium exist in the form of Ni2+ and Cd2+; then add excess NH4HCO3 to the solution to convert cadmium ions into CdCO3 and separate them out in the form of precipitation. Finally, adding NaOH and NaCO3 to the solution converts the nickel ions into nickel hydroxide. Since the solubility product of CdCO3 is much smaller than that of NiCO3, this method can achieve a relatively complete separation of cadmium.
The disadvantage of this process is that NH4HCO3 is easily decomposed automatically, and the operation of this process is very complicated.
4. Nickel-cadmium selective extraction process
(1) Chelating agent extraction. The main steps of the process flow of selective extraction of Ni by chelating agent (Lix64 N or Kelex 120) are: leaching the waste nickel-cadmium electrode material with hydrogen carbonate-pressed ammonia solution (NH4HCO3+NH3·H2O); the concentrations of NH3, NH4+, HCO3- and CO32- in the solution were adjusted by passing through ammonia and carbon dioxide; the leaching solution contained 6g/L of Ni and 5g/L of Cd; Ni was then selectively extracted using an integrator (Lix64 N or Kelex 120).
The presence of cadmium and cobalt ions did not affect the effectiveness of solvent extraction. The ammonia in the raffinate is removed, and the cadmium is converted into a precipitate and removed; after removing the cadmium, the solution is heated to 100 °C, and the cobalt will be converted into a hydroxide precipitate after 1 h. The advantage of this process is that 90% of nickel and 90% of cadmium can be recovered, and the purity of products containing Cd and Ni is also very high. But the disadvantage is that the chelating agent is expensive and the equipment cost is high.
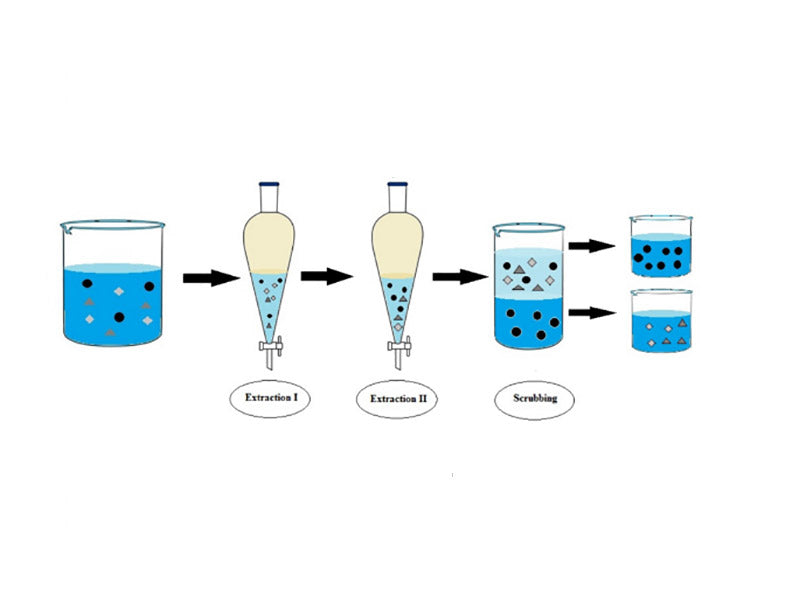
(2) Solvent extraction and recovery of Cd, Co, and Ni. Portugal's Nogueira and others studied the technology of solvent extraction to separate and recover valuable metal components in waste nickel-cadmium batteries. This process overcomes the shortcomings that the main products of the previous wet recovery process are salts and cannot be directly put into the market, and uses a two-step method to extract pure cadmium, pure cobalt and pure nickel from the sulfuric acid leaching solution of waste nickel-cadmium batteries. The first step of the process is to use DEHPA's organic phosphoric acid solvent as the extractant to recover more than 90% of the cadmium; the second step is to use Cyanex's organic phosphoric acid solvent as the extractant to recover more than 90% of the cobalt. This process consumes less energy and has little impact on the environment, and can also be used for the recovery of other types of batteries such as nickel-metal hydride batteries, but the high price of the extractant limits the practical application of this process.
5. Electrolytic recovery process of cadmium
The process of recovering nickel-cadmium battery by electrolysis process: First, the electrode material is extracted from the nickel-cadmium battery by crushing and sieving, and then the active material is dissolved in sulfuric acid. The eluate contained 40 g/L of Cd and 70 g/L of Ni. The leaching solution is electrolyzed, and the metal pot is recovered at the anode, and the purity of cadmium can reach 99.95%. The electrolysis residual solution is mainly Ni, and also contains 3g/L of Cd and 70g/L of Fe. Oxygen or oxidant is added to the residual liquid, the pH value is adjusted to 6 with limestone, and Fe is removed by filtration. Then, gypsum seeds are added to the solution and the gypsum is removed by filtration. When the temperature of the solution is cooled to room temperature, nickel sulfate crystals can be obtained.
Since the electrode potentials of cadmium and nickel are not very different, the current density in the electrolysis is only 7mA/cm2, and the operation process must be very delicate. In addition, the energy consumption of this process is relatively large, which limits the practical use.
6. Active metal replacement process for cadmium
Kaufmann et al. from the former West Germany used a two-step replacement method to separate Cd and Ni. The process flow: first, the waste nickel-cadmium battery was dissolved with sulfuric acid, and the mother liquor contained 10~80g/L of Ni and 120~150g/L of Cd; the temperature is controlled at 25~30℃, 40~100g/L NaCI solution is added to the mother liquor, and the pH value is adjusted to 2.1~2.5; then, active metal aluminum is added to the solution to replace the cadmium.
The chemical reactions that take place in this process are as follows.
2Al+3Cd2+→2Al3++3Cd↓
In the second stage, the temperature of the mother liquor is adjusted to be 55~60°C, the pH value is 2.4~2.5, and the NaCl solution is added to make the concentration reach 120~150g/L. At this time, the active metal Al can replace the Ni. The chemical reaction is as follows.
2Al+3Ni2+→2Al3++3Ni↓
The advantage of this method is that both cadmium and nickel are recovered in the form of pure metals, but the disadvantage is that it is difficult to separate high-purity metals, and the recovered cadmium and nickel cannot be directly used for the production of nickel-cadmium batteries.
7.TNO/ESDEX/LETO process
The main steps of the TNO/ESDEX/LETO waste nickel-cadmium battery recycling process in the Netherlands are: first, crush the nickel-cadmium battery, and then add hydrochloric acid to make the active substances in the battery become ions into the solution;
Finally, an organic solution was added to extract the cadmium. After the extraction, the raffinate is evaporated to obtain NiCl2 crystals, in which HCI can be reused; NaOH or Na2CO3 are added to the extract to convert cadmium ions into cadmium carbonate for precipitation.
TNO/ESDEX/LETO and other commonly used wet processes, such as ESDEX in Canada, HYDROMETAL in Belgium, Filter in the United States and UNINIQUEL in Spain, etc. Due to the high cost of the process, factories only use these methods for recycling certain non-ferrous metals that are more economical than cadmium, rather than recycling technologies specifically for nickel-cadmium batteries. This type of process is usually suitable for sludge and nickel-cadmium battery production waste. Its gas pollutant emission concentration is low, but the water pollutant emission problem is serious, and the secondary pollution is difficult to control.
8.TOHO zinc process
Japan TOHO Zinc Co., Ltd., Mitsubishi Mining Smelting Co., Ltd. and Kan Sai Catalyst Co., Ltd. use waste nickel-cadmium battery or Jin-cadmium battery production waste and battery factory sludge to separate nickel and cadmium.
The main steps of the process are: firstly, the sludge of the battery factory is sulfurized and filtered to obtain cadmium sulfide, and the nickel-cadmium battery is crushed and oxidatively roasted in a rotary furnace. The sulfide filtration residue is neutralized and filtered to obtain nickel carbonate crystals, and the remaining nickel-iron alloys from oxidative roasting can also be sold. This process can be used for the recovery of waste nickel-cadmium batteries and nickel-cadmium battery production waste, and can be combined with crude zinc refining to form a combined high temperature and wet process. The advantage of this process is the high purity of the electrolytic pot, but the disadvantage is the high energy consumption.
9. High temperature recycling process of waste nickel-cadmium battery
The high-temperature recycling process of waste nickel-cadmium batteries is also known as the fire process. The basic principle is to use carbon as a reducing agent to reduce cadmium oxide at high temperature, and then steam and separate the cadmium. The materials from the museum are high-purity cadmium, and the remaining materials are nickel-iron alloys, both of which can be sold as products. The pyrotechnic process can be used not only to treat waste nickel-cadmium batteries but also to treat mixed batteries.

10. BATREC-AG process
Sweden's BATREC-AG is the first company in the world to realize commercial recycling of waste batteries. Its process technology is based on the system developed by Japan's Sumitomo Company, which can recycle more than 95% of the mixed waste batteries, and the processing capacity can reach 3500t/a. The process consists of three basic processes: the shaft furnace is treated at a high temperature of 400~750 °C to decompose the organic part of the waste battery; the high-temperature furnace treats the metal part in a reducing atmosphere at 1500 °C, Zn, Pb and Cd are volatilized, and Fe, Mn and Ni are melted; the metal gas (Zn, Pb and Cd) is recovered by using a splash condenser.
11. SNAM process
The French SNAM company has long started to use the fire process to recycle waste nickel-cadmium batteries. SNAM uses two processes for the separation of metals: recovery of mercury from mercury-containing waste; extraction and other substances from cadmium-containing waste and recycled waste batteries from the production process of nickel-cadmium batteries. SNAM's nickel-cadmium battery recycling process mainly includes three steps: pretreatment, cadmium distillation and finishing.
The process is characterized by high pot recovery. In 1996, the processing capacity of SNAM's waste nickel-cadmium battery was 4000t/a. The sources of waste processed by SNAM are: Germany, France, the United States, the Far East and some other European countries. SNAM is a major European producer of high-purity cadmium and cadmium oxide from waste brocade-cadmium batteries.
12. Inmetco Process
Inmetco is a stainless steel manufacturer that uses high temperature metal recovery (HTMR) to recover Ni, Cd, and Fe from the U.S. special steel industry. It is located in Ellwood City, Western Pennsylvania, USA. It has a high-temperature metal recovery facility and is one of the major companies in the production of recycled materials for the stainless steel industry in North America. At this company, nickel-cadmium batteries are a valuable resource that can be used to refine stainless steel; while the recovered Cd is sent to a Cd recovery workshop for high-temperature recovery or to other zinc-cadmium smelters for further processing. The recovered metal is eventually sold commercially, and its by-product, inert slag, is used to pave roads and produce other building materials.
Inmetco has 4 furnaces for industrial and residential nickel-cadmium batteries put into use. The steps are as follows. The nickel-cadmium batteries are first sorted by hand. For large industrial nickel-cadmium batteries, dry the electrolyte first. Then, the battery is manually or semi-mechanically decomposed, and the positive electrode of the battery is a nickel-iron alloy, which can be directly used to produce stainless steel. The negative electrode of the battery is mainly a cadmium plate, which is directly sent to the distillation furnace to recover the cadmium. The waste KOH electrolyte is used as a pH adjuster for Inmetco's wastewater treatment. For civilian small sealed nickel-cadmium batteries, the main treatment steps are to crush the battery first, then start the thermal oxidation process, and oxidize and incinerate the organic matter in the waste battery under high temperature and oxidizing atmosphere. The remainder is then sent to a still for cadmium recovery. The distillation residue is used to produce stainless steel, while the distillation distillate is used for remelting and casting into cadmium ingots. Cadmium recovered from nickel-cadmium batteries is 99.95% pure and can be used to produce new batteries.
The high temperature metal recovery technology used by Inmetco has been recognized by the U.S. Environmental Protection Agency as the best practical technology for processing metal waste from the specialty steel industry. At the same time, the US Environmental Protection Agency believes that HTMR technology is also the best technology for recycling Jin-cadmium battery waste.
















