The discovery of pure solid ("dry") polymer electrolytes has a history of more than 30 years, but so far, polymer electrolytes are still only in the research stage of the laboratory, which is far from the actual battery requirements. The most significant improvement in the ionic conductivity of polymer electrolytes is achieved by the gelation of electrolytes [such as solutions formed by dissolving lithium salts in organic solvent mixtures (called plasticizers)] in the polymer (typical such as polyacrylonitrile PAN) matrix. Adding a plasticizer not only reduces the crystallinity of the polymer, but also increases the mobility of the polymer segment. Plasticizers can dissociate a larger amount of lithium salt so that a larger number of carriers participate in ion transport. Low molecular weight polyethers and polar organic solvents are the two most commonly used plasticizers.
Gel-type polymer electrolyte was first proposed by Feullade and Perche in 1975, and later further researched by Abraham and colleagues. In this type of electrolyte, the entire system can be regarded as an electrolyte formed by alkali metals and organic plasticizers uniformly distributed in the polymer main network. In such an electrolyte, the polymer mainly exhibits its mechanical properties and supports the entire electrolyte membrane, while ion transport mainly occurs in the part of the liquid electrolyte contained therein. The conductivity of this type of electrolyte is equivalent to that of an organic solution of an alkali metal salt, generally up to 10-3S/cm at room temperature, and does not require the ability of the polymer body to dissolve the salt. The electrical conductivity of gel polymer electrolytes is mainly related to the physical properties of organic solvents, such as dielectric constant and viscosity. Commonly used plasticizers include EC, PC, γ-BL, etc. In order to increase the dielectric constant of the system, a mixture of several plasticizers can also be used. PC is used as a plasticizer to modify the network PEO, and the room temperature conductivity is close to 10-3S/cm. The PAN is modified with a compound plasticizer, the room temperature conductivity reaches 4×10-3S/cm, and the lithium ion migration number is also increased to 0.6~0.7. Using γ-BL as a plasticizer also produces the same effect, and their decomposition voltage is above 4.5V. A new gel polymer electrolyte can be prepared by choosing PEU as the polymer carrier, PC as the plasticizer and modified montmorillonite as the inorganic additive. The electrolyte not only exhibits good mechanical properties, but also exhibits high ionic conductivity. Someone reported a new type of polymer electrolyte. Firstly, EC/PC is embedded in mesoporous SiO2 to obtain a kind of electroactive EC/PC mesoporous SiO2 nanocomposite particles, and then added to PEO-LiClO4 to obtain a composite gel polymer electrolyte. The room temperature conductivity of the electrolyte reaches 2×10-4S/cm, and the mechanical properties are also greatly improved. The main reason for the increase in conductivity is that the electrically "active" EC/PC mesoporous SiO2 nanocomposite particles provide a unique and fast nanochannel for the migration of lithium ions.
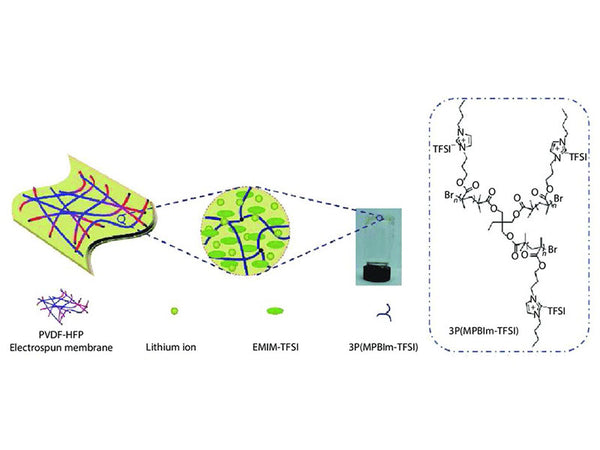
The schematic illustration of gel polymer electrolyte of 3P(MPBIm-TFSI)
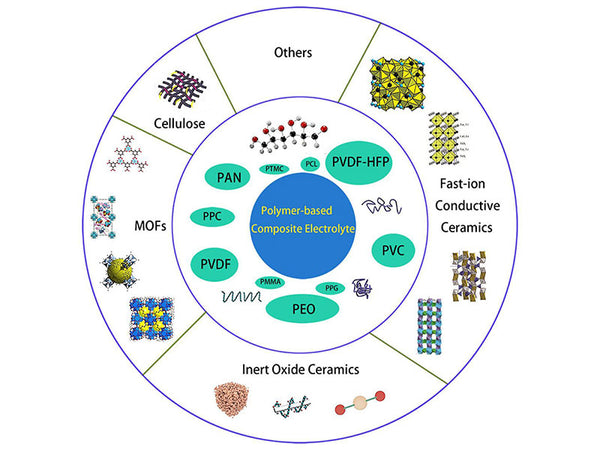
According to the classification of the polymer body, the gel polymer electrolyte mainly has the following three categories.
(1) PAN-based polymer electrolyte
In addition to PEO and its modified polymers, there are many polymer materials that do not contain [CH2CH2O] repeating units on the backbone and side chains, in order to obtain electrolytes with higher room temperature conductivity. The increase in electrical conductivity is a major advantage of PAN-based gel polymer electrolytes instead of traditional solid polymer-based electrolytes, but the gel system is also thermodynamically unstable. After long-term storage, the gel electrolyte may have problems such as solvent leakage, especially in an open environment. This phenomenon is called the shrinkage effect, and it has occurred in many systems. When this effect occurs, the electrolyte solvent will seep out to the surface of the electrolyte membrane, and the electrolyte will gradually become opaque. This change causes the viscosity of the electrolyte to increase, the activity of ions decreases, and the ionic conductivity is therefore significantly reduced.
Dispersing zeolite powder in LiAsF6-PAN gel polymer electrolyte can form a composite electrolyte. It is found that adding zeolite will bring two advantages. One advantage is that the low-temperature ionic conductivity is improved. Although the PAN gel electrolyte is highly disordered, the polymer main chain will rearrange at low temperatures and orientate in a more ordered or crystalline state. Adding a small amount of zeolite particles can prevent this crystallization process from occurring, and retain those amorphous regions that contribute to ion conductivity. In addition to good transport performance, compatibility with electrode materials is also an important parameter to ensure that the electrolyte has acceptable performance in electrochemical devices. When the metal lithium or carbon anode material is in contact with the electrolyte, a thin layer of the third phase is formed between the two bulk phases. When in contact with PAN gel electrolyte, the lithium metal electrode may undergo a passivation process. Another advantage is that the stability of the electrolyte/electrode interface is improved. The addition of 5% zeolite to the gel electrolyte can effectively reduce the growth of the barrier layer on the surface of lithium metal. This favorable interface characteristic is related to the hydrophilic characteristic of molecular sieve. The dispersed zeolite fixes the impurity materials and prevents them from reacting at the interface. Another possible reason for the improved interface properties is that the composite film has a greater viscosity than the gel phase, which prevents the flow of corrosive solvents.
Many reports on the transport of lithium ions in PAN basically indicate that lithium ions have a strong interaction with the polymer, the plasticizer of the polymer backbone, and the polymer backbone. Although the conductivity of PAN-based polymer electrolytes is similar to that of EC/PC liquid electrolytes, measurements of NMR linewidth and spin-lattice relaxation time indicate that the presence of PAN hinders the activity of short-range ions.
(2) PMMA-based polymer electrolyte
In 1985, Iijima et al. first proposed the use of PMMA as a gelation accelerator, and found that the conductivity of the electrolyte obtained by using 15% PMMA with an average molecular weight of 7000 at 25°C can reach 10-3S/cm. Dissolve PMMA with a mass fraction of up to 20% in 1mol/L LiClO4/PC electrolyte at room temperature to obtain a uniform and transparent gel. The conductivity of the gel electrolyte at 25°C is 2.3×10-3S/cm. The addition of high molecular weight PMMA leads to a very high macroscopic viscosity (viscosity of 335Pa·s) without significantly reducing the conductivity of the system. In other words, the conductivity of the system after adding PMMA is still close to that of the liquid electrolyte. It can be considered that PMMA mainly acts as a stiffener, and fast ion conduction is through the formation of continuous conductive channels of PC molecules. The presence of PMMA does not affect the electrochemical stability of the electrolyte.
(3) PVdF-based polymer electrolyte
Due to the strong electron withdrawing group—CF, PVdF—(CH2—CF2—) n-based polymer electrolyte is expected to become a matrix material for polymer electrolytes that are highly stable to anions. As a polymer material, the dielectric constant of PVdF itself (ε=8.4) is also quite high, which helps to dissociate the lithium salt and increase the carrier concentration.
The most critical aspect of PVdF-based polymer electrolyte is its interface stability with metallic lithium. Since lithium reacts with fluorine to generate LiF and F, fluoropolymers are not chemically stable to metallic lithium, and PVdF is not suitable for use in batteries that use metallic lithium as a negative electrode. The conductivity of PVdF-based electrolyte plasticized with PC solution of LiN(CF3SO2)2 is 1.74×10-3S/cm at 30℃, and the oxidation stability potential relative to Li+/Li is 3.9~4.3V. Ion mobility is a decisive factor for polymer electrolytes; adding plasticizers to solid polymer electrolytes can increase conductivity by about 2~4 orders of magnitude.
Due to the excellent properties of room temperature molten salt and polymer electrolyte, a new type of polymer electrolyte can be obtained by mixing 1-butylpyridine bromide with AlCl3 to obtain room temperature molten salt, and then adding a little polypyridine: polybromide 1-butyl-4-vinylpyridine, room temperature conductivity can reach 10-3S/cm. Research has found that when triethylmethylammonium benzoate (TEMAB)/Lithium Acetate (LiOAc)/Lithium bis(trifluoromethylsulfonate) (LiTFSI)=7/2/1, a stable molten salt can be formed at room temperature. The conductivity at 30°C is 10-4S/cm, and the conductivity at 60°C is 10-3S/cm. After a certain amount of PAN is added, the conductivity decreases by 1 to 2 orders of magnitude. Because AlCl3 is sensitive to humidity and unstable in the air, it is cheaper to synthesize the room temperature molten salt with [BF4-] and [PF6-] as the anion, and mix it with polyvinylidene fluoride-hexafluoropropylene (PVdF-HFP) to form a colloidal electrolyte. When the room temperature molten salt: PVdF-HFP=2:1, the room temperature conductivity of the colloidal electrolyte is greater than 10-3S/cm, and at 100°C it is greater than 10-2S/cm, and the open circuit voltage of the single cell is 3.7V.