
Main content:
If you look at your electronics, you'll notice that the lithium-ion batteries they come with lose capacity over time. Once the theoretical cycle number is exceeded, the capacity of the battery will have a very significant decline, and this time it is time to replace the battery. Therefore, lithium battery capacity loss is very important, especially the irreversible battery capacity loss, which is related to the battery life. This article will start from the principle of lithium battery, and introduce the reason for battery capacity loss and irreversible capacity loss.
1.Basic principle of Li ion battery
The process of embedding Li and removing Li between positive and negative electrode materials, which is the charge and discharge process of Li-ion battery.The positive and negative electrode voltage is determined by the relative potential of the material, and the current is determined by the surface area of the crystal involved in the embedding process and the resistance encountered in the process. The capacity is determined by the amount of active substances and how much Li can be embedded and detached.
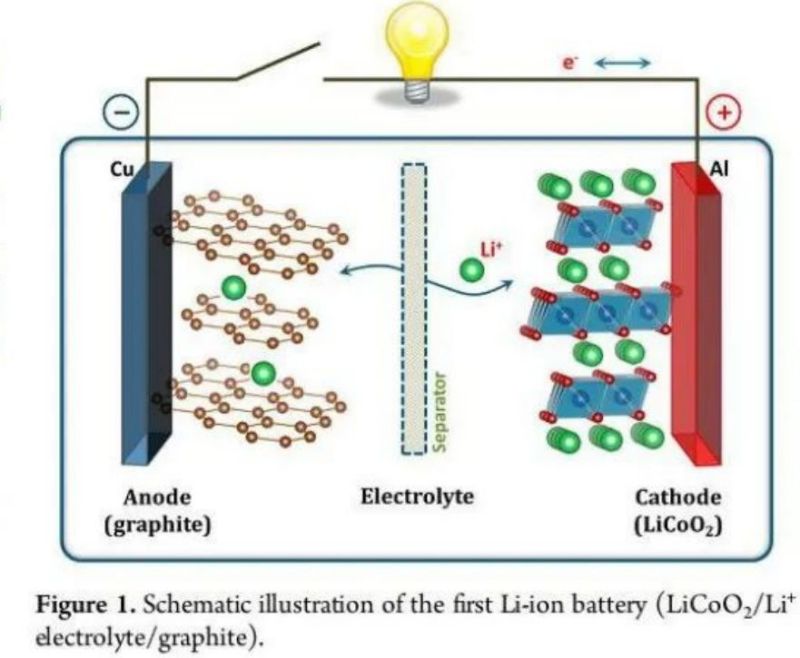
2.Determinants of Li ion battery capacity
- How much is the positive and negative active substance?
- How much Li + is effectively embedded and detached?
- Resistance of reaction process (transfer of electrons and ions)?
- Side reaction and self discharge. Proportion of actual output capacity?
3.Detailed explanation of the causes of Li ion battery capacity loss
① Li-ion battery capacity loss reason-overcharge
- Effect of overcharge on negative electrode
-In the case of overcharge, Li + is easy to be reduced to metal Li when the negative electrode is combined with electrons. The deposited lithium is coated on the surface of the negative electrode, blocking the insertion of lithium, that is, reducing the effective action area of the negative electrode.
-It leads to the reduction of discharge efficiency and irreversible capacity loss.
Reasons:
-Recyclable Li becomes less, and Li reacts with solvent or electrolyte to produce Li2CO3 / LIF and other products that do not contribute capacity.
-Metal Li is usually formed between the negative electrode and the diaphragm, blocking the gap between the diaphragm, increasing the internal resistance of the cell and reducing the actual discharge capacity.
-Consumed by Li reaction generated by electrolyte. This leads to the reduction of discharge efficiency (conductivity reduction) and irreversible battery capacity loss.
- Effect of overcharge on positive electrode
-When the ratio of positive active material to negative material is low, the change of positive electrode plays a decisive role in the influence of cell capacity.
-Overcharge causes the positive electrode to participate in the reaction to produce oxide, resulting in battery capacity loss of positive material, resulting in the decrease of capacity.
Reasons
-The crystal structure of cathode materials has changed and no longer has the function of embedding Li. In essence, some cathode materials react under overcharge conditions to produce other substances.
-The embedded Li function is weakened, resulting in irreversible capacity loss. Effect of overcharge on electrolyte
- Effect of overcharge on electrolyte
-Under high pressure (usually > 4.5V), the electrolyte is easy to be oxidized to form insoluble matter (such as Li2CO3) and gas.
-The insoluble residue blocks the micropores of the electrode and hinders the migration of Li +, resulting in the loss of capacity in the process of circulation.
-The surface area of positive electrode and the types and characteristics of electrolyte additives also affect the oxidation degree of electrolyte under overcharge.
Reasons:
-The ion mobility decreases and the effective area of the electrode is reduced.
-In addition, the insoluble matter generated by the oxidation of electrolyte forms a passive film on the electrode surface, which will lead to the polarization of the electrode, and finally lead to the decrease of the cell voltage, which will affect the actual use capacity of the cell.
②Li-ion battery capacity loss cause-SEI
When the electrolyte and electrode surface are discharged for the first time, a stable and protective solid electrolyte interface (SEI) will be formed. The electrolyte is separated from the electrode to prevent the co intercalation of solvent molecules, but Li + intercalation and de intercalation are allowed.
-SEI formation will consume part of Li +, and the inter electrode capacity balance will be changed, resulting in the reduction of specific capacity. (the capacity loss caused by film formation is related to the type of C used in the electrode, electrolyte composition and additives).
-If cracks occur on the passivation film and solvent molecules penetrate, a part of Li will continue to be consumed. The thickening of the passivation film will block the micropores on the surface of C, affect the normal insertion and detachment of Li, and cause irreversible battery capacity loss in this way.
Avoid:
-Add inorganic additives to the electrolyte, such as C02, n20ac0, etc.
-Organic solvents, such as crown ethers, 12 crown 4 ethers have the best effect.
-Accelerate the formation of SEI and inhibit the co embedding and decomposition of solvents.
③ Li-ion battery capacity loss-electrolyte decomposition

Reduction of solvent
-The electrochemical reaction of PC / EC on graphite produces ch = CH3 (g) / CH2 = CH2 (g) fnlico3 (s), resulting in irreversible battery capacity loss on graphite electrode.
Reduction of electrolyte
-The results show that the insoluble matter produced by the reduction of electrolyte has an adverse effect on the products reduced by solvent. In addition, the electrolyte consumption will change the electrolyte concentration and affect the cell capacity.
Reduction of impurities
1)Too high water content in electrolyte will generate LiOH (s) and Li2O deposition layer, which is not conducive to lithium ion embedding and irreversible capacity loss. 2)CO2 in the solvent can be reduced to CO and LiCO3 (s) on the negative electrode. Co will increase the internal resistance of the battery, while Li2CO3 (s) will increase the internal resistance of the battery.
3)The presence of oxygen in the solvent will also form a part of Li consumed by Li2O.
④ Li-ion battery capacity loss-Self discharge
Reversible capacity loss
-In general, the redox reaction inside the battery leads to electron transfer and consumes part of the capacity, causing battery capacity loss.
-For the specific mechanism, see Chapter 1 basic communication of Li ion
Irreversible capacity loss
-Dendrites penetrate the diaphragm, resulting in micro contact between positive and negative poles, resulting in increased self discharge and loss of available capacity.
-When the positive and negative electrodes are in the charged state, the electrode may have local micro battery action with the electrolyte, and this local action cannot be completely equal at both ends of the positive and negative electrodes, resulting in unbalanced positive and negative electrodes of the cell and irreversible capacity loss.
⑤ Li-ion battery capacity loss reason-electrode instability
Dissolution loss of cathode material
-Structural defects of positive active material; Defects lead to weakening of bond energy and easy fracture and dissolution. In addition, Ni2 generated by dissolution will deposit on the negative electrode and block the micropores, affecting ion embedding and stripping.
-Charging potential too high.
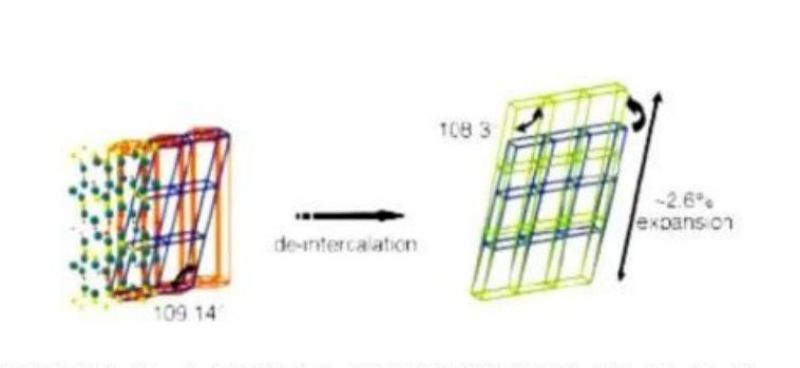
-The content of carbon black in the cathode material. The substances with catalytic properties produced by the oxidation of electrolyte on the surface of carbon black increase the dissolution rate of metal ions. Phase transition (taking lithium manganese oxide as an example)
Phase change
-Overcharge: the cathode material manganese oxide is completely de lithium to form inactive b-mno2.
-Over discharge: the lattice is distorted due to excessive lithium insertion. The cubic spinel structure is a tetragonal spinel structure. The Z axis in the cell is extended by 15% and the X and Y axes are contracted by 6%. After repeated cycles, the positive drawing material will be powdered.
-Note: the negative electrode also involves the change of crystal phase in the process of charging, discharging, lithium intercalation and lithium removal, but it has been considered that the phase change of negative active substances will not cause the capacity attenuation of the battery.
⑥ Li-ion battery capacity loss-fluid collection
Current collector: electrode substrate, metal foil used to collect charge and draw current, positive AI and negative Cu.
-Aluminum is easy to form local corrosion phenomena such as pitting corrosion or pitting corrosion in electrolyte (inhibition: add fluoride).
-Both copper and aluminum are prone to increase the internal resistance of the battery due to the formation of surface oxide film and poor adhesion (inhibition: chromate pretreatment).
-Copper is oxidized to Cu2 + during discharge, diffused to the negative electrode through the electrolyte, and then reduced during charging. Copper dendrites are formed on the surface of the negative electrode, which is very easy to penetrate the diaphragm and cause short circuit (light ones cause the increase of self discharge rate).
-The collector is best pretreated (acid alkali etching, corrosion-resistant coating, conductive coating, etc.) to improve corrosion resistance and adhesion and avoid Li ion battery capacity loss for the battery pack.
4.How to avoid the Li-ion battery capacity loss- A summary
- Prevent overcharge and discharge of the battery.
- Add additives to the electrolyte to reduce side reactions and reduce the loss of solvent and solute.
- Considering the loss of additional active substances, electrolyte and electrolyte, additional supplement shall be made in the manufacturing stage.
- Pretreat the electrode and collector to prevent additional increase of internal resistance.
















