
Peter von Moshbruck and his Leiden Flask
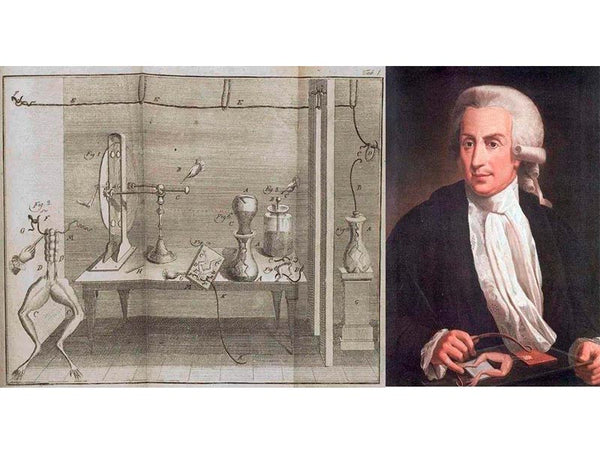
Records show that von Krist first discovered between 1745 and 1746 that electricity could be stored in glass jars filled with copper wire or mercury. Almost at the same time, Professor von Moshbruck accidentally dropped a charged nail into a glass bottle while doing an electrical experiment. He thought that it would not take long for the electricity charged on the nail to escape. However, as soon as he touched the nail, he suddenly felt an electric shock. After repeating the experiment several times, he found that the electricity could be stored by placing an electrified object in a glass bottle. This is the prototype of the famous "Leyden bottle". Later, scientists continued to use the Leiden bottle to carry out various experiments and a large number of demonstration performances. Among them, the Frenchman Nolette performed the most spectacular performance in front of a cathedral in Paris. Nolette invited the royal family of Louis XV to watch the Leiden bottle performance. He asked 700 monks to line up hand in hand in a 900-foot-long (about 275 meters) line, and the monks at the front held the Leiden bottle with their hands. The monk at the end of the row held the lead of the bottle. At the moment of contact, 700 monks jumped up almost at the same time because of the electric shock, and everyone present was dumbfounded. In the same year, Franklin in Philadelphia, USA, received a Leyden bottle sent from England. He was very interested in this, and made a series of experiments using the Leiden jar, and discovered the laws of positive and negative charges and the conservation of charge. The invention of the Leiden bottle provides an effective way to store electricity for human beings, and is the cornerstone of the development of battery technology.
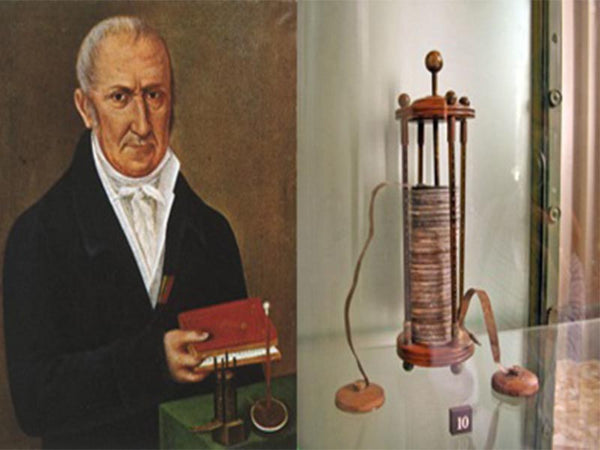
Alexander Volta
In 1800, Volta made the earliest battery in human history, which later generations called the Volta battery. Its appearance is an important milestone in the development of batteries. A voltaic cell consists of a series of circular zinc and silver sheets that overlap each other, each pair of silver and zinc sheets separated by cardboard dipped in salt water or other conductive solution (electrolyte) The current loop of the battery. At that time, the French emperor Napoleon I preferred scientific development. After learning that Volta invented the battery, he immediately summoned Volta and asked him to demonstrate relevant experiments in person. Napoleon I awarded Volta a prize of 6,000 francs and medals after watching it, and issued commemorative gold coins. The unit of voltage was also named after Volta, which is still in use today.
In 1830, Sturgeon solved the problem of weak current and polarization in voltaic batteries. The battery he invented consists of a zinc rod wrapped with mercury in a cast iron shell, and many thick paper sheets are placed in the middle to insulate the zinc rod and other metals. The electrolyte of the battery is dilute sulfuric acid. . By using a combination of mercury and zinc, the life of the battery is extended. In 1836, referring to the principle of voltaic battery, Daniel put a copper plate at the bottom of a glass jar, poured half a jar of copper sulfate solution, then suspended the zinc plate in the jar, and finally added zinc sulfate solution, and invented a new type of battery to improve battery performance. The stability of the voltaic battery. Later generations called this kind of battery the Daniel battery, and it was the first battery that could continuously supply power for a long time.
The Grove cell is an early galvanic cell consisting of an anode zinc rod in a dilute sulfuric acid solution and a cathode platinum rod in concentrated nitric acid, separated by a porous ceramic bottle. Between 1840 and 1860, the early telegraph system in the United States mainly used Grove batteries as a power source. Grove batteries have higher discharge current and output voltages about 70% higher than Daniel batteries. However, the Grove battery releases nitrogen dioxide during charging, which is a toxic gas that is hazardous to human health. At the same time, Grove batteries have poor stability and limited use, and were later replaced by Daniel batteries.
In 1859, Plante invented a rechargeable battery capable of producing a very large current. The battery consists of two thin lead plates, separated by rubber, wound into a spiral shape, and then dipped in a 10% sulfuric acid solution. However, due to too few active reactants on the cathode plate of the battery, the amount of stored electricity is limited. Around 1881, one of Plant's assistants, recognizing the importance of lead oxide, improved the battery. He painted red lead on the surface of the lead plate, and then used flannel as a separator, which greatly increased the capacity of the battery. Later, this kind of battery introduced the perforated lead plate and the use of lead-antimony alloy, which made the adhesion of the active material on the lead plate better. Plant rechargeable lead-acid batteries paved the way for the development of electric vehicles.
"Wet" battery by Leclan Hsieh
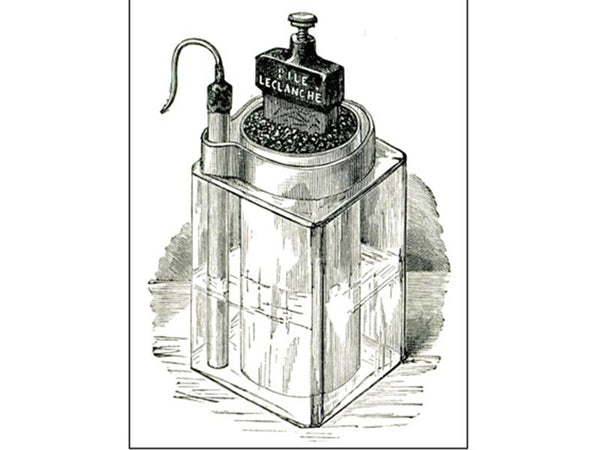
In 1866, Leclanché invented a new type of "wet" battery (zinc-carbon battery). The positive electrode of the Leclanché battery consists of manganese dioxide powder with a small amount of carbon powder, and the negative electrode consists of a piece of carbon inserted into a porous canister to collect the current. The electrolyte used is an ammonium chloride solution. The Leclanché battery was rather bulky and leaked easily, and Tippert improved it in 1881 by encapsulating the negative electrode in a zinc cup along with a porous bottle, and applied for a patent. On this basis, in 1887 Carl Gessner applied for a patent for dry batteries and commercialized dry batteries for the first time. The technology uses zinc as the negative electrode and packaging casing, the electrode is wrapped in a porous material, and the top of the battery is sealed for easy portability and transportation.
In order to reduce the internal resistance of the battery, Belgian chemist Julien added mercury to the lead-antimony alloy in 1888, which enhanced the conductivity of the lead mesh, thus greatly reducing the internal loss of the battery and prolonging the life of the battery.
Vadema Jonger's nickel-iron battery
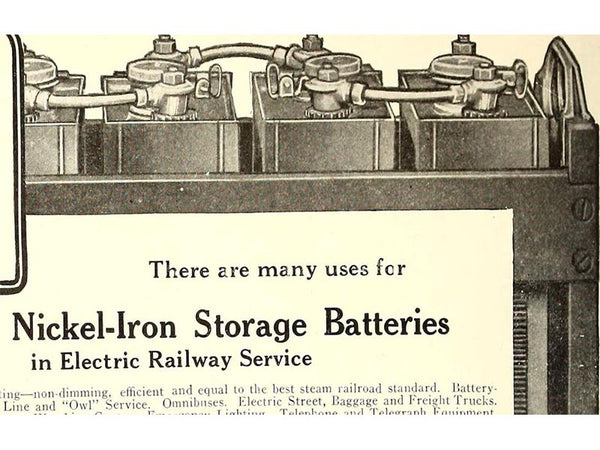
At the same time, Jonger invented nickel-cadmium batteries, nickel-iron batteries and alkaline Zn-MnO2 batteries, and first used nickel plates. Unfortunately, the practical application of these alkaline batteries was greatly limited because the plate materials of these alkaline batteries were much more expensive than those of other batteries at the time.
















