
Main content:
1.The basic principle and technology of lead-acid battery
Lead-acid battery technology (invented by Plante in 1859) is currently the most widely used electrical energy storage technology, and has made significant progress since its inception. The energy cost of lead-acid battery is the lowest among current battery energy storage technologies, which makes it possible to continue to have market space for many years to come. Lead-acid batteries can be divided into two categories based on the properties of their electrolytes.
Liquid electrolyte batteries have a long service life, but require regular and frequent maintenance. Some batteries can be maintained every 200 to 250 discharge cycles, or the equivalent of once a year.
The basic materials of lead-acid battery are lead and sulfuric acid, which are composed of a negative plate composed of porous lead and a positive plate composed of lead oxide. Electric vehicles mainly use lead-acid batteries. In traditional lead-acid batteries, in order to prevent the positive and negative plates from short-circuiting, a cellulose diaphragm is used between the positive plate and the negative plate in the single cell to ensure isolation. In high-performance cells, microporous separators are used to prevent any possible short-circuit failure.
The terminal voltage of a single lead-acid battery is about 2V, and the terminal voltage can vary between 1.7V and 2.4V according to the state of charge during its normal operation.
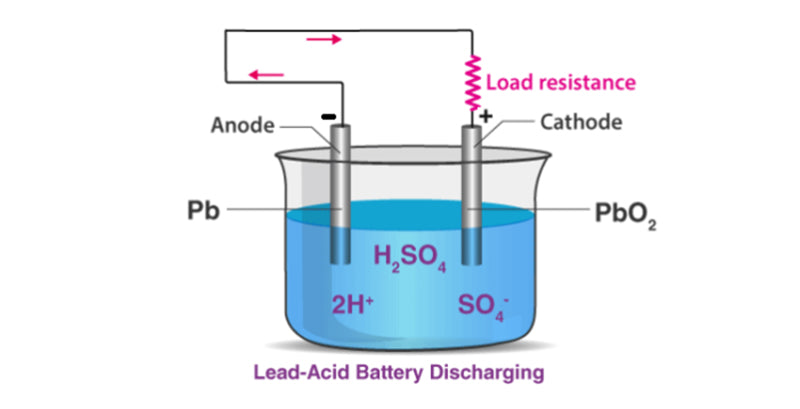
The electrochemical reaction equations that take place on the electrodes of lead-acid battery are given below:
Lead-acid battery anode (oxidation reaction):
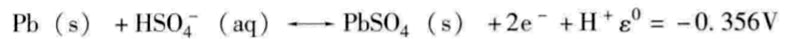
Lead-acid battery cathode (reduction reaction):
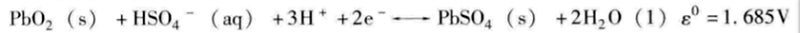
Maintenance-free lead-acid battery can use two different electrolytes, a silica gel and a non-woven fiber membrane. Maintenance-free lead-acid battery is achieved by equipping a regulating valve to remix the gases. Using this technology, the lead-acid battery is organically combined with the charger to ensure that the lead-acid battery is in the best working condition. It should be emphasized that maintenance-free lead-acid batteries have weak overcharge resistance. The energy density of this type of lead-acid battery is 35~50W·h/kg. The "two-pole" battery is a new concept that replaces the assembly of positive and negative electrodes in conventional batteries, resulting in superior performance. This type of lead-acid battery has a cycle life of about 500 cycles at 80% depth of discharge.
There are many lead-acid battery manufacturers around the world, and almost all of them participate in the battery performance improvement program initiated by the Advanced Lead-Acid Battery Association (ALABC).
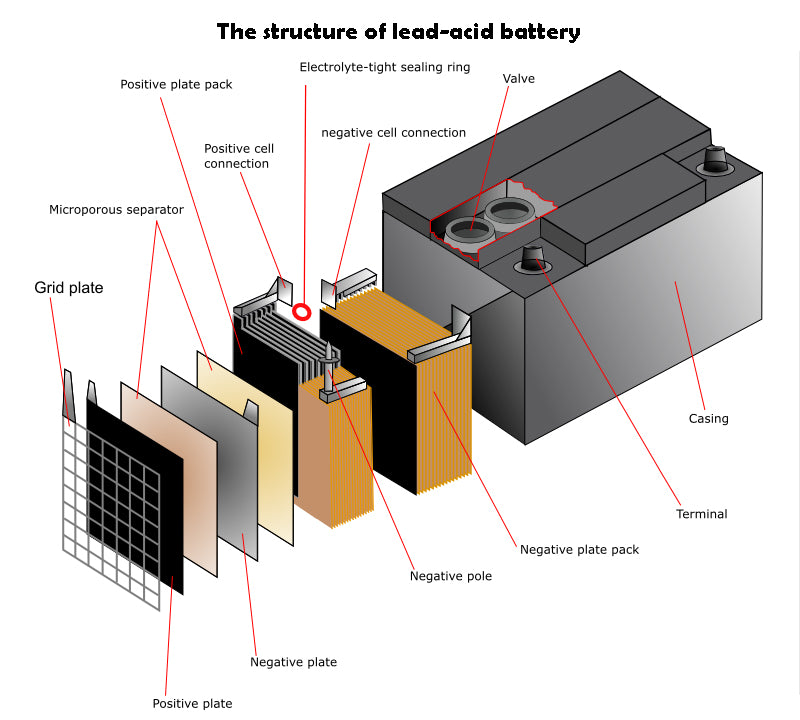
Lead-acid battery can be divided into four main "subcategories" as follows
1) Start the lead-acid battery (grid grid plate), which is produced in many countries in the world, and this lead-acid battery can be seen everywhere. They are designed to remain fully charged at all times, allowing for rapid high-current discharges when needed. They have a high self-discharge rate, are relatively inexpensive (about 0.2 EUR/kW·h in terms of available capacity), and are widely used.
2) Drive lead-acid battery (flat plates), used in electric vehicles and more common automatic running locomotives (railways, elevators). They are designed for one charge-discharge cycle per day. Therefore, it can be discharged to a lower state of charge. Its price is relatively high, about 0.5 EUR/kW·h in terms of available capacity.
3) Colloidal electrolyte lead-acid battery (flat plate), mainly used for traction occasions. Its capacity is usually small, about 100A h. This type of lead-acid battery does not require any maintenance, can run continuously for 3 to 5 years, and costs about 0.6 euros/kW·h based on the available capacity. They are also often used on small specialized platforms (such as wireless communication stations), especially mobile devices (such as flashing or illuminated buoys). A "waterproof" battery has also emerged, in which the liquid electrolyte is sucked in and permanently stored in a bag of synthetic material.
4) Fixed (static) lead-acid battery (tubular plates), derived from the need for reliable power supply technology (communication relay stations, etc.). This type of lead-acid battery is designed to be continuously charged with a very small current (i.e., float charge to keep the battery voltage at a constant value), with a low self-discharge rate (1%~2% per month), and when needed , can be fully discharged. Even so, lead-acid battery can undergo charge-discharge cycles and continue to operate at a relatively low state of charge for several weeks. Although this type of lead-acid battery requires less maintenance (adjustment of the water level) and the cost is slightly lower (0.5 EUR/kW h), it still has a high performance index (lifespan can reach 8~12 years), so It can be widely used in battery energy storage power station above 300A.h.
In fact, all lead-acid batteries cannot continue to discharge below 20% of their rated capacity. Otherwise, the electrodes will be sulfidized (see the next subsection), resulting in a loss of lead-acid battery capacity, an increase in internal resistance, and a decrease in available capacity.
2.State-of-charge control of lead-acid battery
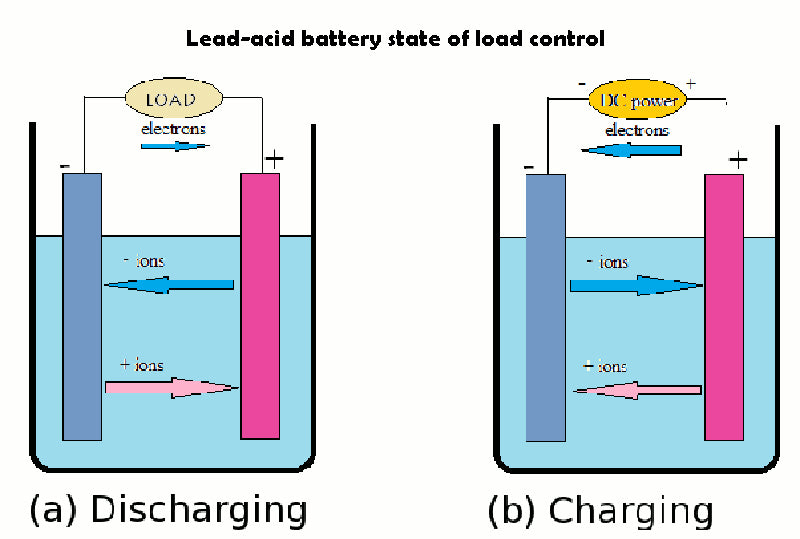
When the lead-acid battery is discharged, the concentration of sulfuric acid in the electrolyte decreases continuously, and when it is charged, it will react again to form sulfuric acid. The state of charge of the battery can be determined by measuring the electrolyte concentration, which can be achieved by measuring the acidity. After the lead-acid battery is fully charged, if the charging current continues to be applied, the water near the electrodes will be electrolyzed, producing hydrogen and oxygen, which are eventually released as gases. If overdischarged, or if the sulfuric acid concentration is not controlled, the sulfuric acid can corrode the electrodes and produce lead sulfate that is difficult to remove, causing lead-acid battery to fail. Therefore, in order to keep the lead-acid battery in a good state, it is very important to check the charging or discharging state at all times. Overcharging or overdischarging will cause damage to the battery to a certain extent.
3.Self-discharge rate and battery life of lead-acid battery
The self-discharge rate depends on the material used in the lead-acid battery (eg lead alloy, separator, etc.). For lead-acid battery with lead antimonide electrodes (this alloy can improve the mechanical strength of the electrodes), the monthly self-discharge rate is on the order of 10%; Lead-acid battery, at a temperature of 20 ° C, the monthly self-discharge rate can be reduced to about a few percent, but lead-acid battery of this material is relatively fragile. The self-discharge rate varies greatly with temperature. A relatively close statement is that the self-discharge rate follows Arrhenius' law, that is, the self-discharge rate doubles for every 10 °C increase.
Assuming over-discharge protection, and by limiting the depth of discharge per day (<15% NC) and the depth of discharge per quarter (<60% NC), the service life of lead-acid battery can reach 6 to 7 years. Among them, NC represents the rated capacity of the battery.
Read more: Limited life of lead-acid batteries
















