The electrochemical synthesis method is a green synthesis method. This method can be used for the preparation of polymer lithium ion battery cathode materials, and also for the preparation of oxide materials such as LiCoO2.
The electrochemical synthesis of organic compounds is called organic electrosynthesis. It is an interdisciplinary subject involving electrochemistry, organic synthesis and chemical engineering. In 1834, Farady claimed that certain hydrocarbons could be obtained by electrolyzing acetic acid. This was the organic electrosynthesis reaction later known as the "Kolby reaction"
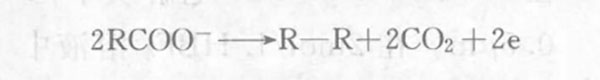
Compared with traditional organic synthesis, organic electrosynthesis has significant advantages: electrochemical reactions are realized through the gain and loss of electrons on the electrode by the reactants. In principle, no other reagents need to be added, which reduces material consumption and environmental pollution; the selectivity is very high, the side reactions are reduced, the product purity and yield are high, and the product separation and purification work is greatly simplified; the reaction is carried out under normal temperature and pressure or under low pressure, which is very beneficial to save energy and reduce equipment investment; the process flow is simple and the reaction is easy to control.
Due to the theoretical advancement of organic electrosynthesis technology and the rapid development of modern electrochemical science and technology in the 1960s, the industrialization process of electrosynthesis has been greatly promoted. In 1965, the 150,000-ton dinitrile plant of Monsanto in the United States was completed and put into operation, which marked that organic electrosynthesis had entered the industrialized era, and the classification method of organic electrosynthesis was more complicated.
According to the types of organic reactions that occur on the electrode surface, it can be divided into two major types of organic electrosynthesis reactions, namely, the anodic oxidation process and the cathodic reduction process. The anodizing process includes: electrochemical oxidation reaction; electrochemical halogenation reaction; anodizing reaction of benzene ring and side chain groups of benzene ring; anodizing reaction of heterocyclic compound; anodizing reaction of nitrogen-containing sulfide. Cathodic reduction processes include cathode dimerization and cross-linking reactions; electro-reduction of organic halides; electro-reduction of hydroxyl compounds; electro-reduction of nitro compounds; electro-reduction of fluorine-based compounds.
According to the position and role of electrode reaction in the whole organic synthesis process, organic electrosynthesis can be divided into two categories: direct organic electrosynthesis reaction and indirect organic electrosynthesis reaction. Direct organic electrosynthesis reaction, that is, the organic synthesis reaction is completed directly on the surface of the electrode; indirect organic electrosynthesis reaction, that is, the oxidation (reduction) reaction of organic matter is carried out by traditional chemical methods, but the oxidizing agent (reducing agent) can be regenerated and recycled using electrochemical methods after the reaction. Indirect electrosynthesis can be operated in two ways: in-tank and out-of-tank. In-tank indirect electrosynthesis is to conduct chemical synthesis and electrolysis in the same device, so this device is both a reactor and an electrolytic cell. The out-of-tank indirect electrosynthesis method is the electrolysis of the medium in the electrolytic tank, and the electrolyzed medium is transferred from the electrolytic tank to the reactor.
Electrochemically active polymers are composed of molecules with conjugated electronic structures. Its preparation revolves around how to form the structure of free electrons or holes that have the ability to migrate inside the molecule. Polyaniline can be prepared by electrochemical polymerization. The electrochemical polymerization method uses the electrode potential as the initiation and reaction driving force of the polymerization reaction. The polymerization reaction is carried out on the surface of the electrode and the polymer is directly formed.
(1) Preparation of polyaniline electrode material. Electrochemical methods such as galvanostatic method and potentiostatic method can be used to polymerize aniline in water-soluble electrolyte. The electricity used is HClO4, HCl, H2SO4, CF3COOH and HBF4. The polymer is electrodeposited on the matrix material, such as Au, Pt, stainless steel, SnO2 sheet and carbon and other materials.
During electrochemical polymerization, polyaniline first forms a dense spherical structure, and then forms a porous fiber structure. When the acidic electrolyte and the current density are 1mA·cm2, aniline can be electropolymerized and deposited to form a light porous sponge with a thickness of several millimeters. At high current density (5mA·cm2), fibrous polyaniline is formed with a diameter of about 0.1μm. The polyaniline obtained by galvanostatic polymerization in 0.5mol/L H2SO4 electrolyte is in a spherical state with a diameter of about 0.5pm. At 2mol/L HBF, the radius of the porous polyaniline fiber synthesized by the constant current in the solution decreases with the increase of the current density, and the porosity of the film remains about 50% unchanged. When 0.25% (mol fraction) of o-phenylenediamine is added to the acidic aniline solution, a dense cross-chain structure polymer is formed.
The electrochemical polymerization of aniline was studied in an organic medium. Scanning electron microscopy (SEM) studies showed that the surface film of polyaniline formed under high acid conditions was smooth and flat. Compared with the aqueous electrolyte product, the electrochemical performance of the film obtained by the non-aqueous medium is not much different, but the polymerization reaction of the non-aqueous system can effectively remove the water from the material. Fiber-reinforced porous polyaniline can be used as an electrode material for lithium-ion batteries.
The layered structure of polyaniline cathode material exhibits the properties of an inner and outer double layer. Inorganic counter ions are located in the inner layer, and organic polymers are mainly distributed in the outer layer to counter anions.
The research trends on the preparation of polyaniline mainly focus on the development of its composite material, including the exploration of inorganic active materials and organic active materials with good compatibility. By adding certain conductive fillers or adhesives, the mechanical properties and processability of polyaniline can be improved.
(2) Synthesis of polythipan. Using electrochemical reduction method, such as in acetonitrile solution, using Ni(ph)2+Br2 as a catalyst, electrochemically reducing 2, 5-dibromothiophene, polythiophene can be obtained on the cathode. This method is also known as the cathode synthesis route. However, since the obtained polythiophene is in a neutral state, that is, an insulating state, the electrode surface is quickly passivated, and the thickness of the obtained film does not exceed 100 nm. This method can be used for electrode corrosion protection.
Through the electrochemical oxidation method, the polythiophene film can be prepared directly from the thiophene monomer. This method is also known as the anode synthesis route. This method has the following characteristics: the method is simple and can be used for mass preparation; the film is directly formed, and the prepared polythiophene film is in a conductive state. The performance of the polythiophene film strongly depends on the solvent used, the nature of the supporting electrolyte, and the specific electrochemical method used. The solvent used must have a large dielectric constant to ensure the ionic conductivity of the solution. At the same time, the solvent is electrochemically inert at a higher potential (1.4~2.3V, vs. SCE). It has been reported that 3-methylthiophene can be polymerized in aqueous solution. However, the presence of trace amounts of water is quite harmful to the electrochemical oxidative polymerization of thiophene, which greatly reduces the effective conjugated chain length and conductivity of polythiophene. Studies have shown that the introduction of water causes the introduction of carbonyl groups on the polythiophene main chain, thereby destroying the conjugated structure. Most polythiophenes are stored in strictly anhydrous, high dielectric constant, low nucleophilic aprotic solutions, such as acetonitrile, benzonitrile, nitrobenzene, and acrylic carbonate. The current efficiency of electrochemically polymerized thiophene in these solutions is very high. The supporting electrolyte used must also be low nucleophilic, such as ClO4-, BF4-, CF3SO3-, etc. The nature of the doped ions has an impact on the morphology and electrochemical properties of the prepared polythiophene. When CF3SO3- is used as the doping ion, the poly-3-methylthiophene prepared in acetonitrile solution even shows a certain degree of crystallinity. In the experiment, if the specific electrochemical oxidation method is different, the properties of the obtained polythiophene film are also very different. The methods used are constant current method and constant potential method, and other methods include cyclic voltammetry and current pulse method.
Electrochemical polymerization of thiophene in conventional solutions has the following disadvantages that are difficult to overcome: the oxidation potential of the polymerization process is relatively high, exceeding the peroxide potential of polythiophene, which inevitably causes the synthesized polythiophene product to peroxidize and cause its degradation. The obtained polythiophene film has low strength, which limits the direct application of the film. Therefore, some people used boron trifluoride ether system in the electrochemical polymerization of thiophene, and successfully reduced the polymerization potential to 1.2V [vs.Ag/AgCl (3.5mol/L, KCl)] to avoid the peroxidation of polyphene. The tensile strength of the resulting polyphene film even exceeds that of metallic aluminum. At the same time, the method is used for electrochemical synthesis of poly-3-alkylthiophene, and the obtained films all show high strength. Furthermore, it was found that the obtained polythipan and poly-3-methylthipan films all showed high conductivity anisotropy, and their conductivity parallel to the film direction was 104 times that of the direction perpendicular to the film.
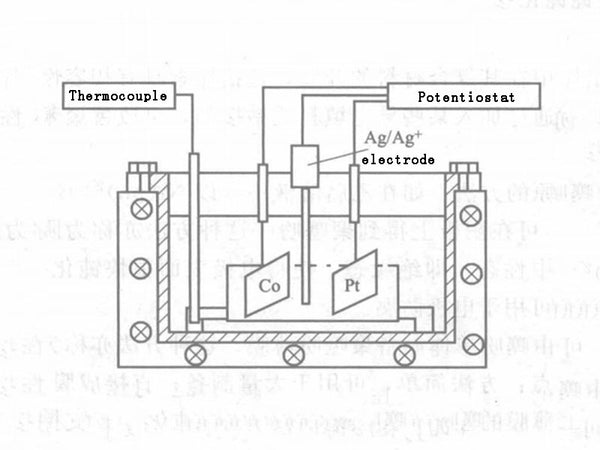
(3) Electrochemical synthesis of LiCoO2. Using Co flakes, cobalt nitrate, Pt flakes and LiOH as raw materials, Co and Pt flakes are taken according to 10mm × 10mm × 0.2mm and then mechanically polished. After ultrasonic cleaning in acetone, chromic acid treatment for 16h, then ultrasonic cleaning with double distilled water and drying. The cobalt nitrate was prepared into a 0.5mol/L solution, and then the cobalt nitrate solution was added dropwise to a 1mol/L sodium hydroxide solution to obtain a blue cobalt hydroxide precipitate. After the precipitation is filtered and washed, 4~6mol/L LiOH solution is added to prepare a suspension, that is, an electrolyte.
The electrolyte is placed in a closed reactor, the working electrode and the reference electrode are immersed in the solution and connected to the positive and negative electrodes of the power supply respectively, as shown in Figure 1. The control reaction parameters are as follows: the current density is 0-10mA, the reaction temperature is 60-200℃, the reaction time is 0.5-48h, and the reaction pressure is the saturated vapor pressure at different temperatures. Anyway, brown powder appeared at the bottom of the beaker after the end, and there was also a brown film on the surface of the Co electrode. The powder and film are washed and dried to obtain the product. The powder is composed of chrysanthemum-shaped crystal grains with a particle size of about 0.2~0.4μm. Electrochemical tests show that the material exhibits good cyclic voltammetry performance.