main content:
Hydrogen energy refers to the chemical energy contained in the hydrogen element (H). As an energy source, it may be unfamiliar to everyone. With the intensification of the oil crisis, many people think of hydrogen energy and have high hopes for it. In nature, almost all hydrogen elements exist in the form of compounds, mainly in water and hydrocarbons, which are inexhaustible and inexhaustible. However, to extract hydrogen from the compound as an energy source, it needs to be exchanged with other energy sources. It seems that we have to do this, because hydrogen energy is indispensable from the needs of energy development.
1 Hydrogen has special functions
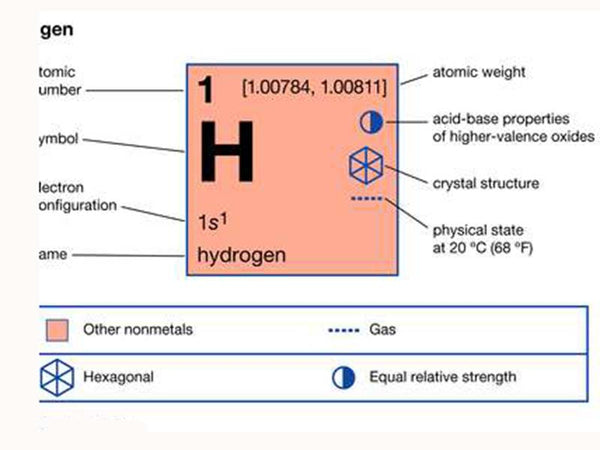
Hydrogen is the first element in the periodic table and the lightest element. To say that it is light, first of all means that its atom is light, with only one proton and one electron, and the atomic weight is only 1. As a simple substance, hydrogen is also particularly light, with a density of 0.0695 to air. Hydrogen is relatively stable. It is inactive at room temperature and does not dissolve much in various liquids. However, it is different in high temperature situations. Hydrogen is very active and can combine with oxygen to generate water and release heat. Therefore, hydrogen is also A fuel. Hydrogen combines with a variety of metals and non-metals to form compounds. Hydrogen is very "tenacious" and difficult to liquefy. The "critical temperature" of liquefaction is -239.9°C; the critical pressure (the minimum pressure required for liquefaction) is 12.8 atmospheres. Before reaching the critical temperature, no matter how much pressure is applied, it will not liquefy. After liquefaction, it will quickly evaporate under reduced pressure or elevated temperature. These properties of hydrogen make it special.
From an energy point of view, hydrogen is a clean and efficient fuel. Hydrogen is burned to produce water, no pollutants are emitted, and no greenhouse gases such as carbon dioxide are emitted. The calorific value of hydrogen is very high, nearly three times that of gasoline, which is the highest among all fossil fuels, chemical fuels and biofuels. Except for nuclear fuels, the calorific value of all fuels cannot be beaten. This high-density energy of hydrogen happens to be an ideal fuel for aerospace power. The powerful power of satellite launch rockets is liquid hydrogen as fuel. The use of hydrogen as an energy source for "fuel cells" is another magical effect, opening up a new era in which hydrogen is used as a power fuel instead of petroleum, and the future is boundless.
Hydrogen energy is different from "movement energy" such as electricity, water energy, and wind energy, and is a "carrier energy" like general fuels, that is, it is a substance that can provide energy. Therefore, it also provides a new way of energy storage, which can convert surplus or cheap electric energy into hydrogen energy through the electrolysis of water. Hydrogen can be stored and adjusted like natural gas and gasoline, unlike electricity that must be produced and used immediately. With the development of nuclear energy and renewable energy, the amount of electricity produced will also increase, and there may even be a surplus of electricity. Converting the surplus electricity into hydrogen energy is a complementary method.
2 How hydrogen can replace petroleum

The total number of automobiles in the world today has exceeded 600 million. Automobiles have become the main body of ordinary transportation and logistics. The entire civilized world cannot do without automobiles. The automobile industry is so developed and has become a pillar industry of the multinational economy, and it is inseparable from the credit of oil. Since the invention of the automobile, the automobile has depended on oil to survive. More than cars, all means of transportation, including ships, airplanes, and even trains, rely on oil. Legend has it that the "oil king" (referring to oil companies in general) once issued a "spell": Without oil, cars will become a pile of scrap iron. However, the whole world has been fitted with "wheels", the world can be without oil, and it can no longer be without cars.
Who can crack the spell of the oil king? Hydrogen energy! It can take on the historical mission of replacing oil. General Motors Corporation of the United States even put forward the concept of "hydrogen economy", which aroused the resonance of many scholars and politicians. It is understandable that in the United States, the energy consumption in the transportation sector is close to 40% of the country's total energy consumption, more than industry and any other sector. There are many, almost equal to the total annual oil consumption of the United States. It is not difficult to understand that the oil crisis is simply a fatal threat to the US economy. In the eyes of Americans, oil and transportation have become almost synonymous with economy. If hydrogen energy can be used to carry out a revolution in automobile fuels, the impact of oil on the entire economy can be alleviated.
The general method of replacing petroleum with hydrogen as a vehicle fuel is to slightly modify the combustion system of the internal combustion engine and directly replace gasoline with liquid hydrogen. However, it is also difficult to store liquid hydrogen on-board and prevent liquid hydrogen from leaking. The most feasible way is to use a hydrogen fuel cell to convert hydrogen into electrical energy so that the electric motor drives the car.
Schematic diagram of Zafir-Hydrogen One powered by hydrogen fuel cell
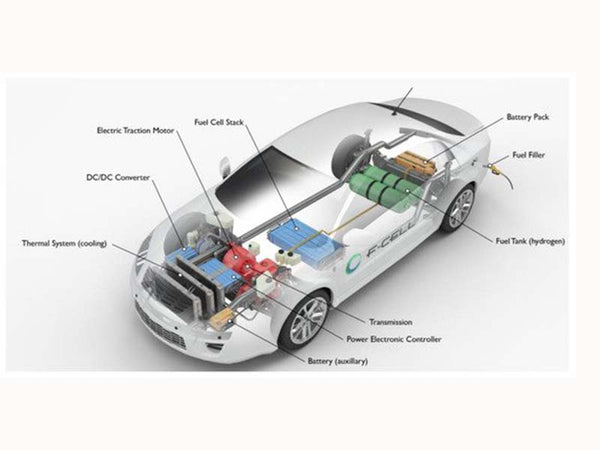
In this way, the vehicle-mounted hydrogen storage device will be more flexible. In 2000, General Motors successfully launched the "Hydrogen Power No. 1", marking the entry of hydrogen fuel cell technology into a practical era. "Hydrogen 1" uses liquid hydrogen on the vehicle to provide fuel. The overall size of the battery part is roughly the same as the power part of an ordinary car. The power of the battery pack can reach 80-120 kilowatts, which can make a car with a mass of 1.5 tons reach 140 kilometers per hour. Liquid hydrogen storage The fuel stored in the cylinder allows the car to travel continuously for 400 kilometers. The appearance of "Hydrogen 1" is similar to a typical 2.4-liter diesel car. The fuel cell stack can be started at a low temperature of -40°C, which is difficult for ordinary cars to do.
|
Tips: Hydrogen Fuel Cell
|
In recent years, almost all major automobile companies such as General Motors, Chrysler, Mitsubishi, Nissan, Honda, Volkswagen, and Mark have rushed to announce plans to develop hydrogen fuel cell vehicles, and have launched prototype vehicles on display, aiming at the market to step up development. It seems that in the early part of the 21st century, the industrialization of hydrogen fuel cell vehicles is a foregone conclusion. Within 20 to 30 years, hydrogen fuel cell vehicles on the world market are expected to surpass gasoline vehicles and become the dominant vehicle type in the market. Automobiles will also enter a pollution-free era.
Automobiles do not need oil, and hydrogen energy can be used in industries and related departments that originally needed oil, let alone. The world economy will not decline because of oil depletion, and the environment will become better because of reduced pollution.
3 Overcoming hydrogen storage difficulties

As a powerful fuel, hydrogen has enabled mankind to realize the dream of flying to the sky, visit the moon and other planets, turn ancient myths into reality, and enable mankind to open up a vast space in the universe. Hydrogen fuel will also effectively become "man-made oil" and better replace the exhausted fossil energy. It is indeed a high-strength "martial arts" and unlimited "goods". However, hydrogen also has inherent shortcomings and is very "stubborn". Hydrogen is very light, has a huge unit mass, is difficult to liquefy and is easy to leak, and it is very inconvenient to store and carry. As a fuel for vehicles, hydrogen storage devices will occupy a large ratio of the limited space on the vehicle and reduce the transportation capacity of the vehicle. This is another problem that needs to be solved, and it is also the focus of hydrogen storage technology.
China launches lunar exploration satellite "Chang'e-1"

According to the characteristics of hydrogen, there are generally three ways to store hydrogen on vehicles, namely liquefaction, compression, and hydride generation. The conditions can be reversed during use.
The storage method of low-temperature liquefied hydrogen is to freeze the hydrogen to -254°C, which becomes liquid hydrogen, and stores it in an insulated container with a vacuum on the partition wall. This "insulated container" can effectively prevent heat conduction, convection and radiation, and is similar to the principle of a household thermos bottle, ensuring that the hydrogen is in a low-temperature liquefaction state. In this way, the liquid hydrogen container can be placed on the car like a car fuel tank, and it occupies a small volume. Of course, this storage method is costly, and thermal insulation and safety technologies are also more complicated. Liquid hydrogen storage has been widely used in space rockets, but it will take time for it to be widely used in automobiles. The key technology for liquid hydrogen storage is the insulated container. A kind of "microbead insulation material" has been developed, made of silicon dioxide, the hollow wall is thin, the size is micrometers, and some microbeads are coated with aluminum film, which has the function of blocking heat radiation. The thermal conductivity of the microbeads is extremely small, which can completely inhibit the heat convection between the microbead particles. The microbeads are filled into the partition wall of the container to form a new type of thermally insulated container, which does not need to be vacuumed and has better thermal insulation effect. It is an ideal liquid hydrogen storage device and is now widely used in space flight.
The compressed hydrogen storage method means that hydrogen is compressed and stored in a steel cylinder. This is a common method for storing gas. Obviously, the storage capacity of this method is very limited. Under a pressure of 20 MPa (equivalent to 200 atmospheres) at room temperature, the hydrogen storage capacity of ordinary steel cylinders only accounts for 1.6% of the mass of the steel cylinder. Driving a certain distance continuously requires about 4 kilograms of hydrogen in the car, which is equivalent to 48.83 cubic meters of hydrogen under normal pressure, and the calorific value is equivalent to 10.5 kilograms of gasoline. Isn't it too small? But this already means that the mass of the cylinder has reached 250 kilograms. Using lighter composite materials with more pressure-resistant gas cylinders and increasing the pressure to fill the gas can make the quality of hydrogen storage reach 3% to 6% of the quality of the gas cylinder, and the manufacturing cost of such gas cylinders is very high.
Metal hydride hydrogen storage method uses the principle of reversible reaction between hydrogen and metal hydride to store hydrogen. The so-called hydrogenated metal refers to the metal that can undergo hydrogenation. It is actually a variety of metal alloys such as lanthanum-nickel alloy, iron-titanium alloy and other hydrogen storage devices. The hydrogen storage device contains solid metal hydrides. Hydrogen is decomposed when heated; on the contrary, hydrogen is stored in a cooled state. In this way, the pressure of hydrogen storage, charging combination and heating release is below 1 MPa (equivalent to 10 atmospheres), which is easy to operate and safer. However, due to the high density of the alloy, the amount of hydrogen storage is relatively small, accounting for only 1% to 2% of the mass of the hydrogen storage device.
In addition to the above-mentioned general technologies, people have now developed a variety of promising technologies, such as the "Carbon Nano Tube" adsorption technology. The hydrogen storage capacity may account for 10% of the mass of the hydrogen storage device. The hydrogen storage technologies of "microsphere" materials are all developing in the direction of high hydrogen storage quality, safety, convenience and practicality. It is foreseeable that once the vehicle-mounted hydrogen storage technology achieves a major breakthrough, the replacement of gasoline-fueled vehicles by hydrogen-fuel vehicles will be fully realized, regardless of whether internal combustion engines or hydrogen fuel cells are used.
4 What kind of energy is used in exchange for hydrogen energy

Energy can be converted to each other, whether it is fossil energy or renewable energy, primary energy or secondary energy, can be used to convert into hydrogen energy. So, what kind of energy is used to convert into hydrogen energy, or what method is used to produce hydrogen Woolen cloth?
Hydrogen has long been widely used, especially as a chemical raw material for the refining of the fertilizer industry and oil refining products, not for fuel. Traditional large-scale hydrogen production uses coal, petroleum and natural gas as raw materials, or as a by-product in the production of caustic soda by electrolysis of aqueous salt solutions. However, in the long run, it is not advisable to exchange fossil energy for hydrogen energy. The development of hydrogen energy is mainly to replace petroleum. Of course, it is not necessary to use petroleum to produce hydrogen, and other fossil raw materials cannot last for long. Using electricity to produce hydrogen through electrolysis of water is the most vital, because there are many sources of electricity, which may gradually become surplus and cheap. Hydrogen can also be produced by using biomass such as straw as a raw material, and hydrogen can also be produced by electrolysis of water according to local conditions. Since the hydrogen and oxygen that make up water are very tightly combined, it costs a lot of electricity to decompose them, and the efficiency is not very high. Even at a pressure of 5 MPa (equivalent to 50 atmospheres), the electrolysis efficiency is only 50% to 70%. However, when electricity is surplus, for example, using part of the electricity to produce hydrogen near a solar power station is a feasible way.
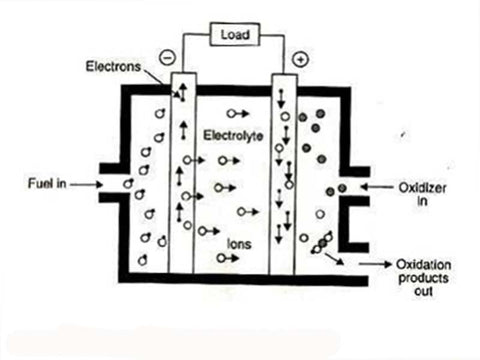
Schematic diagram of alkaline aqueous solution electrolysis cell
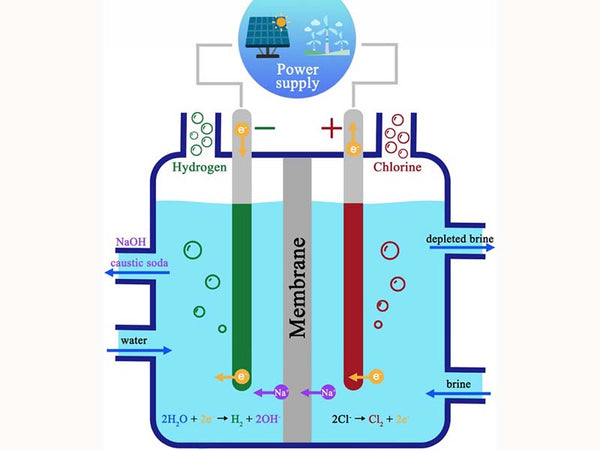
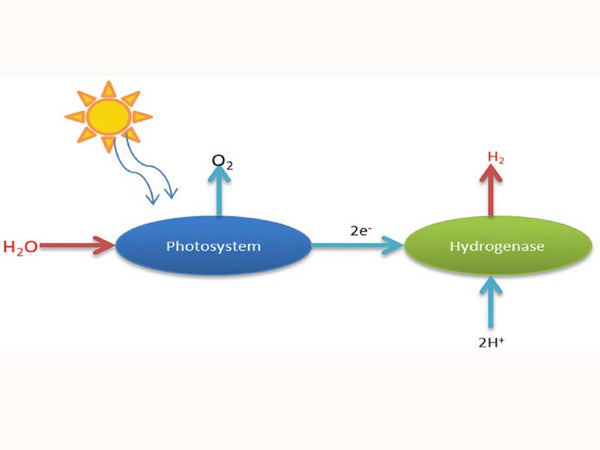
A method similar to hydrogen production by electrolysis is called solar photochemical decomposition of water to produce hydrogen. It also uses electricity, but because it directly uses part of the solar energy, it saves much effort. Different from the direct electrolysis of water, the photochemical decomposition of water first uses the energy of sunlight, and in the presence of a photosensitive catalyst, the water is ionized into hydrogen ions (H+) and hydroxide ions (OH-), which are then obtained through electrochemical reactions. Hydrogen and oxygen. This consumes much less electricity and can be produced at a lower temperature. This method can also become the basis for large-scale hydrogen production.
Schematic diagram of photoelectric catalytic direct decomposition of water to produce hydrogen
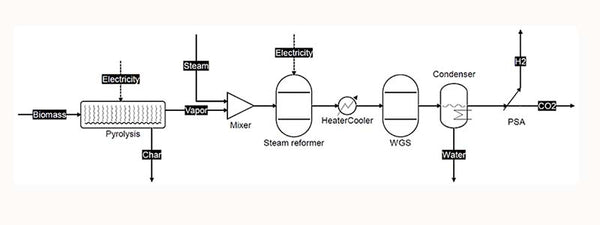
Hydrogen production from biomass is a technology that is being vigorously developed. Construct engineering devices in areas rich in crop stalks and other biomass resources, and input the stalks into water vapor at high temperature to generate syngas. The main component of syngas is hydrogen, and the carbon dioxide in it is removed through conversion and purification to obtain high-purity hydrogen. And because water participates in the reaction, the hydrogen production is higher than the hydrogen content of biomass, reaching 150%, which is very cost-effective.
Schematic diagram of the process flow for hydrogen production by catalytic vaporization of biomass
5 Talk about methanol

Here we go back and talk about methanol, because its separation and combination with hydrogen has important utilization value. Hydrogen can be converted into methanol, and methanol can also be converted into hydrogen. The change between "anti-hands" has achieved the unique function of methanol, and has become an indirect and convenient method of hydrogen storage, so that people do not have to worry about hydrogen storage. First of all, let's talk about the "change" of methanol into hydrogen. The most commonly used method for producing hydrogen from methanol is catalytic steam reforming. The chemical reaction formula is as follows:
CH3OH+H2O=CO2+3H2
Through the purification device, carbon dioxide and other impurity gases can be removed to obtain high-purity hydrogen. This reaction is suitable for small and medium-scale hydrogen production. A portable methanol reformer can be assembled to adapt to a portable fuel cell as a hydrogen source, and the operation is flexible. In this way, methanol can be installed in the fuel tank of a car like gasoline and indirectly become the hydrogen source of hydrogen fuel cell vehicles without worrying about insufficient hydrogen storage on the vehicle.
Let's talk about the production of methanol. I said earlier that large-scale production of methanol is synthesized from hydrogen and carbon monoxide. Methanol comes from fossil fuels. Now it is used to produce hydrogen. Why do you need to do this? Yes, it really doesn't make sense to do so. However, just as ethanol does not necessarily have to be produced from food, methanol does not have to be fossil fuel. Biomass waste can also be used to synthesize methanol through gas production, without increasing carbon emissions during their use. Hydrogen produced from biomass is used as a special energy source for mobile devices, which solves the difficulties of vehicle energy sources, which makes sense. Furthermore, methanol can be synthesized from hydrogen and carbon monoxide, can it be made from hydrogen and carbon dioxide? This is an interesting question. If possible, CO2 waste becomes a resource that can be recycled, so that methanol does not increase carbon emissions during use. The answer is yes. I understood the reaction of methanol steam reforming to produce hydrogen, and now I repeat it again:
CH3OH+H2O=CO2+3H2
This is a reversible reaction. If the conditions of the reverse reaction are met, methanol can be produced from hydrogen and carbon dioxide. Carbon dioxide is available almost everywhere, as long as there is hydrogen, methanol can be made. However, no one has done this yet, and there are many people who have studied it. It's not that this is unattainable, it's all about taking some energy as a price. People are still willing to do this when the oil crisis situation is compelling. As a fuel for automobiles, methanol is "tender like water". Whether it is used as an internal combustion engine or a fuel cell, it is convenient and safe. It does not have to face the "stubborn" hydrogen directly. This may also be another good solution.
Knowing various energy sources, people will find an interesting phenomenon. With the development of energy history, the carbon content in fuel is gradually decreasing, while the hydrogen content is gradually increasing. In terms of the chemical composition of the fuel, the ratio of hydrogen to carbon atoms in the molecule is roughly as follows:
Firewood (1:10)~(1:3)
Coal 1:2
Petroleum 2:1
Natural gas 4:1
Hydrogen 2: 0
Is it because carbon-generated greenhouse gases are annoying and are gradually eliminated? No, people know that the greenhouse effect is not so early. Humans have regarded charcoal as a treasure since ancient times (charcoal is one of the forms of carbon, and carbon also exists in various compounds, especially hydrocarbons). It is closely related to people's lives and is indispensable. People are pursuing fuels with high calorific value and high efficiency, while coal, oil, natural gas and hydrogen satisfy people's desires. One by one is valued more and more, objectively making people's main fuels gradually away from carbon. Nowadays, it is the greenhouse effect that makes people realize the necessity of staying away from carbon fuels and limiting carbon emissions. Coupled with the pressure of the oil crisis, they have to put forward the proposition of a "low-carbon industry and a "low-carbon society". As an efficient and clean energy hydrogen fuel Being able to emerge at the historic moment is the product of advancement in science and technology. Hydrogen energy will develop vigorously, with a bright future, and mankind will inevitably enter the hydrogen fuel era.