Coal, oil, and natural gas are resources that can directly provide energy. They have a long development time and mature development technology. They can be mined in large quantities and widely used. They have high energy density, are convenient to transport and store, and are relatively safe to use. They are easier to be processed and converted into secondary energy sources that are more suitable for actual use, such as coal gas, coke, steam, electricity, gasoline and hydrogen. Therefore, the three fossil energies of coal, oil and natural gas are worthy of the three pillars of modern energy.
main content:
- 1.Coal is the driving force of the industrial revolution
- 2.How is coal formed?
- 3.How much coal resources are there?
- 4.Century of Petroleum
- 5.Petroleum is the "twin brother" of coal
- 6.rising star natural gas
1 Coal is the driving force of the industrial revolution
Coal was discovered and used by mankind a long time ago. China was the first country in the world to use coal, and there have been records of coal in the Han Dynasty. At that time coal was used not only as fuelwood, but also as a fuel for boiling salt and making iron. After the Han Dynasty, coal was called "carbon coal", and people had accumulated a wealth of experience in finding coal mines. During the Yuan Dynasty, the Italian Marco Polo discovered the use of coal in China and called coal "burnable black stones", which was spread to Europe as an anecdote. The Europeans' use of coal only began on the eve of the Industrial Revolution.
In the middle of the 18th century, the industrial revolution that began in Britain was based on the invention and use of the steam engine. It started with the cotton textile industry, and then drove various industrial sectors to realize the transformation from manual production to machine production, and finally replaced the human part with machines. Manual labor has made human society move from an agricultural society to an industrial society. At that time, machine production was driven by coal. Since then, the coal industry and mining industry have developed rapidly. Coal has replaced the fuelwood that has been used by humans for thousands of years and has become the main energy source for life and production, thus changing the slow development of human society and enabling rapid economic and social development.
For a long time after the Industrial Revolution, coal has been the most important energy source in society. Until 1965, coal still occupied the first place in the world's energy production and consumption, accounting for about 42% of total energy consumption. Since then coal gave way to oil, and production declined rapidly. By 2000, the world's total coal production was only 3.1 billion tons, accounting for only 25% of the world's total energy consumption.
2 How is coal formed?
Why is it said that coal is a fossil energy source? Here it is necessary to understand how coal is formed. Coal is a mineral formed from the remains of plants over a long period of time. This kind of long time is counted in units of billions of years. Since the formation of the earth, let alone the beginning of life, geologists have divided the 600 million years ago into Paleozoic, Mesozoic and Cenozoic according to the main stages of biological evolution. Several "epochs" and "worlds" (see table). The “stone coal discovered now” was formed around the Cambrian of the Early Paleozoic, more than 600 million years ago, while the main coal types, anthracite, bituminous and lignite, were formed around the Late Paleozoic to the Mesozoic Jurassic and Cretaceous. Before and after, it has been more than 100 million to 400 million years ago. Plant remains are piled up and converted into coal through two stages of peatification and coalification. Peatification refers to the complex biochemical changes and physics of higher plant remains. The chemical change becomes the process of "peat." Coalification is divided into two stages, "diagenesis" and "metamorphism". The diagenesis stage makes peat form lignite, and the metamorphic stage is lignite advances to form bituminous coal under higher ground temperature and formation pressure. anthracite.
|
era |
age |
generation |
Elapsed time/billion years |
The period when organisms start to reproduce |
Main types of coal |
||||
|
plant |
animal |
||||||||
|
New generation |
Quaternary |
Holocene Pleistocene |
0.03 |
Angiosperms multiply and become coal to provide raw material |
The emergence of ancient humans |
peat |
|||
|
Tertiary |
New Tertiary |
Pliocene Miocene |
0.25 |
Angiosperm |
Mammals appear |
peat Brown carbon Low metamorphic bituminous coal |
|||
|
Old Tertiary |
Oligocene Eocene Paleocene |
0.08 |
|||||||
|
Mesozoic |
Cretaceous |
Late Cretaceous Early Cretaceous |
1.40 |
Angiosperms and gymnosperms are extremely prosperous, providing the original material for coal formation |
Reptiles appear |
Brown carbon bituminous coal |
|||
|
Jurassic |
Late Jurassic Middle Jurassic Early Jurassic |
1.95 |
|||||||
|
Triassic |
Late Triassic Middle Triassic Early Triassic |
2.30 |
|||||||
|
Paleozoic |
Late Paleozoic |
Permian |
Late Permian Early Permian |
2.70 |
Gymnosperms and spore plants are extremely prosperous and provide coalOriginal substance |
Amphibian emergence |
bituminous coal anthracite |
||
|
Carboniferous |
Late Carboniferous Middle Carboniferous Early Carboniferous |
3.20 |
|||||||
|
Devonian |
Late Devonian Middle Devonian Early Devonian |
3.70 |
Fish appear |
anthracite |
|||||
|
Early Paleozoic |
Silurian |
Late Silurian Middle Silurian Early Silurian |
4.40 |
Ferns and seaweed proliferate in large numbers, providing the original material for the formation of rock coal |
Invertebrate emergence |
anthracite |
|||
|
Ordovician |
Late Ordovician Middle Ordovician Early Ordovician |
5.00 |
Stone coal |
||||||
|
Cambrian |
Late Cambrian Middle Cambrian Early Cambrian |
6.20 |
|||||||
|
Proterozoic |
Sinian Period |
Late Sinian Middle Sinian Early Sinian |
appointment16 |
Bacteria and algae |
|
||||
|
Early Proterozoic |
20 |
||||||||
|
Archaic |
|
45 |
No coal
|
||||||
Coal is an organic mineral. The organic matter is mainly composed of four elements: carbon (C), hydrogen (H), oxygen (O), and nitrogen (N). There are also some inorganic substances in coal, mainly sulfur (S) and phosphorus. (P) and rare elements, etc. Carbon is the main component of coal and the most important combustible substance. Its content increases as the degree of "metamorphism" of coal is deepened. For example, the carbon content in peat is 50% to 60%, and that of lignite is 65% to 75. %, 75%~90% in bituminous coal, 90%~98% in anthracite. Hydrogen is also an important combustible substance in coal. It has a high calorific value. The calorific value of hydrogen of the same mass is 4 times that of carbon. The content of hydrogen in coal decreases as the degree of coal metamorphism deepens, and it also has a lot to do with primitive coal-forming plants. Coal formed by lower plants such as algae has a high hydrogen content, some exceeding 10%. Sulfur is the main harmful substance in coal. It exists in the form of inorganic sulfur and organic sulfur. Most of the inorganic sulfur exists in the form of pyrite (FeS2). Sulfur generates sulfur dioxide (SO2) acid gas during the combustion of coal, which becomes a pollutant of the atmospheric environment. Phosphorus is also a harmful component. It is transferred to pig iron through coking and coke use, which seriously affects the quality of pig iron and makes pig iron brittle.
Different types of coal have different coal quality. Commonly used coal quality indicators include water, ash, volatile components and calorific value. The amount of moisture content depends on the internal structure of the coal and the external environment. Coal with high moisture content is not conducive to combustion. Ash is the residue after coal is completely burned, mainly from external minerals and sandy soil. High ash is not only unfavorable for combustion, but also increases the difficulty of soot treatment. Volatile matter refers to the liquid and gas decomposed from the organic matter in the coal when the coal is heated to about 850°C under the condition of isolating the air. Coal with a greater degree of deterioration, such as anthracite, has less volatile content and is more difficult to ignite and burn. Obviously, coal with more moisture and ash has a lower calorific value. In order to facilitate the conversion of the energy per unit mass of coal, the calorific value of standard coal is artificially specified, or the calorific value is 7000 kcal/kg (kcal, that is, kilocalorie, is a commonly used non-statutory unit, which is converted to the legal unit joule) See "Common Energy Measurement Units and Conversion" for the relationship). The quality indicators of these coals need to be mastered during use.
| Tips: the calorific value of the link fuel The unit mass of solid, liquid fuel or unit volume of gas fuel is completely burned, and the heat released when the combustion product is cooled to the temperature before combustion is called the calorific value (or "calorific value") of the fuel, and the unit is kJ/kg or kJ/m3. The calorific value is divided into high calorific value and low calorific value. The high calorific value refers to the heat released when the fuel is completely burned and the water vapor in the product is condensed into water; the low calorific value only refers to the heat released when the fuel is completely burned, excluding the heat released by the condensation of water vapor into water Heat (latent heat of vaporization of water). Because when the fuel is burned in a combustion device such as a boiler, the original water in the fuel and the water generated after hydrogen combustion are exhausted with the flue gas in a vapor state, which is difficult to use, so the low calorific value is closer to the actual available fuel calorific value . In the thermal calculation, the calculation basis is based on the low calorific value. |
3 How much coal resources are there?
Coal is ubiquitous all over the world, and its distribution is relatively even. It is also easy to understand this distribution characteristic from the origin of coal. According to relevant calculations, by 2000, the world's proven coal reserves equivalent to standard coal was approximately 2.1 trillion tons, of which the recoverable volume was approximately 830 billion tons. In other words, the proven existence of coal does not mean that all coal can be mined. Due to objective conditions and mining technology restrictions, not all coal has mining value. With the growth of coal production and consumption and the strengthening of transportation infrastructure, there will be more The coal resources become mineable resources. In countries and regions with strong growth in coal production, the proven reserves of coal are still increasing because they continue to explore and make new discoveries while they are producing. For example, Australia, Indonesia, the United States, Canada, Colombia, China, India and other countries have this situation.
China’s coal resources are very rich. The discovered reserves are more than 1,000 billion tons, and the verified reserves are more than 700 billion tons, of which the recoverable reserves are more than 110 billion tons, second only to the Commonwealth of Independent States (240 billion tons) and the United States. (240 billion tons), ranking third in the world. China's coal has many coal formation ages, is widely distributed, and has a complete range of coal types. High-quality coal with low sulfur content and high and medium calorific value accounts for the majority. In 2000, China's coal production exceeded 1.4 billion tons, making China the world's largest coal producer. Compared with oil and natural gas resources, coal occupies an important position in China's energy resources and also dominates in total energy consumption. Until 2000, it still accounted for 67%. Even if this ratio will be reduced in the future, the pattern of using coal as the main energy source will not change in a relatively long period of time. This is also the weakness of China's energy structure.
4 century of oil
Petroleum was discovered very early. China was one of the first countries to discover, exploit and utilize oil. It was recorded in the "Book of Han Geography" written by Ban Gu more than 1900 years ago. Oil. "Petroleum" was first named by the famous Northern Song Dynasty scientist Shen Kuo in "Mengxi Bi Tan". In China's history, oil has been used for lubrication, lighting fuel and medicine, and it has also been used in military fire attacks very early. The Chinese people have always been in the forefront of the world in the use and exploitation of petroleum resources, and they have accumulated a wealth of knowledge and valuable experience. However, due to political reasons and the aggression of foreign powers, China's oil industry has fallen into a dying situation. In Europe and the Middle East, there have long been some oil and gas sprouts, which were once regarded as miracles and attracted many tourists, which gave rise to "Zoroism". In the latter part of the 19th century, Russia and the United States successively drilled oil-producing wells. It was already a remarkable thing to extract "kerosene" from oil for lighting purposes. This period is called the "kerosene age".
The above shows that the discovery and use of oil has a long history, but the large-scale exploitation in the world was a matter of the 20th century. At this time, on the one hand, the discovery of a large number of oil fields, on the other hand, the superior performance of petroleum in application. In 1885, American geologist LC White published the "Anticline Theory" (a doctrine about the geological structure of oil reservoirs). In the following decades, numerous oil and natural gas fields have been discovered all over the world, making the world's crude oil production more expensive. It has greatly increased, from an annual output of 20 million tons in 1900 to 500 million tons in 1950. At the beginning of the 20th century, the extensive use of internal combustion engines promoted the vigorous development of the petroleum industry, and many factories needed petroleum products for their engines and automobiles. Before 1940, oil was mainly used to refine gasoline, which can be called the "gasoline age". After 1940, in addition to providing power fuels for various sectors, the development of the chemical industry also used petroleum products as basic raw materials. More than 3,000 products made from petroleum have penetrated into various fields of the national economy, such as synthetic plastics, synthetic rubber and synthetic fibers, fertilizers and medicines, etc., which have become common products in society, and the amount of oil used for chemical raw materials has been increasing. Therefore, the petroleum industry has developed into the "fuel and chemical raw material era". Because of the excellent combustion performance of petroleum, especially petroleum products are indispensable in transportation, it has brought huge profits to petroleum production, and the number of petroleum exploitation and applications has increased sharply.
In the 1960s, under the competition of cheap oil from the Middle East, oil rose rapidly in the world's primary energy consumption structure. In 1966, oil surpassed coal to become the world's number one energy source for the first time, reaching 54% of total energy consumption. In 1980, world oil production reached 3 billion tons, and in 2000 it reached 3.55 billion tons, accounting for 40% of total energy consumption.
It can be said that since the oil industry became an important industry on a global scale in the early 20th century, oil has changed everything in the world. It has derived almost all modern industries. The 20th century is therefore called the "century of oil." Become the "black gold that dominates this world."
5 Petroleum is the "twin brother" of coal
Oil and natural gas were produced in the same age as coal, and their sources are all evolved from the remains of ancient organisms, which can be described as "twin brothers." Due to different biological sources and differences in geological conditions, during the long-term changes, three different types of minerals, coal, oil, and natural gas, have been generated.
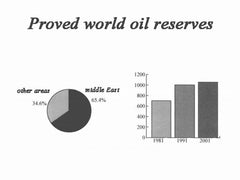
During the formation of these three types of minerals, some came from the same "semi-finished products". However, the generation of oil is not as common as coal. It has two basic conditions-water and basin. Under these two conditions, it is conducive to the deposition and "soilification" of bacteria, plankton organisms and higher plants, and underwater organic matter is transformed into hydrocarbons under reducing conditions. It is precisely because of the limitation of oil-forming conditions that oil deposits are very unevenly distributed in the world. According to relevant statistics in 1999, of the world’s total proven and recoverable oil reserves of more than 300 billion tons, the Middle East accounted for 65.4%, Latin America accounted for 8.6%, Europe accounted for 8.3%, Africa accounted for 7.2%, and North America accounted for 65.4%. 6.2%, and the Asia-Pacific region accounted for 4.3% (Figure).
Petroleum is different from solid coal. It is a liquid combustible mineral composed of various hydrocarbons (hydrocarbons) and a small amount of impurities. The density is between 0.75 and 1.00 at room temperature. The chemical elements that make up petroleum are mainly carbon and hydrogen, followed by sulfur, nitrogen, and oxygen. Generally, the carbon content in petroleum accounts for 84% to 87%. The hydrogen content accounts for 11% to 14%, and both appear in the form of hydrocarbons, accounting for 97% to 99% of the total oil content. Due to the high hydrogen content, the calorific value of oil is much higher than that of coal, and the standard oil calorific value is set at 10,000 kcal/kg. The total content of sulfur, oxygen, nitrogen and trace elements only accounts for 1% to 4%; in individual cases, due to the high content of sulfur, the total content of impurities may be as high as 3% to 7%. The trace elements in petroleum constitute ash, but in general, petroleum has very little ash. Sulfur is one of the most important elements in petroleum, and its content in petroleum varies greatly. It is based on elemental sulfur (S), hydrogen sulfide (H2S), mercaptans (RSH), sulfides (RSR′), and disulfides (RSSR). ') and other forms. Sulfur is also a harmful impurity in petroleum. It is easy to produce compounds such as hydrogen sulfide, iron sulfide (FeS) or sulfuric acid, which can cause serious corrosion to metal equipment such as machinery, pipelines, refinery towers, and oil tanks. If used as fuel oil, it will produce SO2. Acid gas pollutes the atmosphere. Therefore, the sulphur content is a main indicator for evaluating the quality of oil. Generally, oil with a sulfur content of more than 2% is called "high-sulfur oil", and oil with a sulfur content of less than 0.5% is called low-sulfur oil." Strict desulfurization measures must be taken during the refining process and when burning petroleum.
The five main elements of carbon, hydrogen, sulfur, nitrogen, and oxygen constitute a huge variety of compounds in petroleum, so petroleum is also a valuable raw material for the chemical industry.
6 rising star natural gas
Like the discovery and exploitation of oil, natural gas was discovered by mankind very early. China is also the first country in the world to develop gas fields. The "Zi Liujing" gas field in Sichuan has been exploited for more than 2,000 years, and the deeds of natural gas boiling salt are well-known in the world. By the end of the Song Dynasty in the 13th century, people had been able to extract shallow natural gas from artesian wells on a large scale.
The so-called "natural gas" does not generally refer to all natural gases in nature, but refers to combustible gases generated by organic substances deposited in the stratum. Natural gas is a mineral and can be exploited as an energy source. Generally speaking, natural gas is related to the deposition and changes of biomass in the strata in ancient times. It is the "three twin brothers" with coal and oil. Natural gas has more extensive generation conditions than petroleum, and some are associated with petroleum, called "oil-type natural gas", which belongs to "wet gas", which is generated by the cracking of petroleum at a higher earth temperature. Some are independent pure gas reservoirs, called "pure natural gas", which belongs to gas. It is the gas produced during the transformation of organic matter from the immature stage to the mature (stable) stage under the action of biochemistry. Some co-exist with coal. "Coalbed methane", commonly known as gas, is the gas generated during the coalification process of coal seams. Some are combined with water and called "natural gas hydrate", which is solid and commonly known as "combustible ice." Although there are many types of natural gas, they are usually The natural gas mentioned only refers to the first two categories, namely oil-based natural gas and pure natural gas, which are called "conventional natural gas". The output of the two categories currently accounts for 60% and 40% of the total output respectively. The main component of natural gas is gaseous hydrocarbons, mainly methane. The methane content in "pure natural gas" often reaches more than 95%, and its calorific value is below 8,870 kcal/m3 (methane calorific value); in oil-type natural gas, there are more heavy hydrocarbons, with a higher calorific value, up to 20,000 The calorie is cubic meters. In general, natural gas has a high calorific value and is a high-quality energy source. There are other components in natural gas, such as nitrogen, carbon dioxide, carbon monoxide, hydrogen sulfide, hydrogen, and trace inert gases, with varying contents. These Gas will affect the quality of natural gas, especially the harmful element sulfur will pollute the environment. However, compared to coal and oil, natural gas is a relatively clean energy source.
Natural gas is rich in resources in the world, with proven reserves reaching 400 trillion cubic meters to 600 trillion cubic meters so far. Calculated by calorific value, it is basically the same as oil resources. With the development and utilization of natural gas, the proven reserves of natural gas are still increasing.
Since natural gas is an excellent energy source with high calorific value, relatively clean, and abundant resources, why is it “rising up” instead? Natural gas also has its own shortcomings. Because it is a gas, it is difficult to store and transport, has a large investment in production, and has a long investment recovery cycle. It is vulnerable to rejection and inhibition of other energy production, mainly oil production. In the early and mid-twentieth century, due to its superior performance, especially as a power fuel that is difficult to replace in transportation, oil promoted its vigorous production and development. The convenient storage, transportation and use of oil and its low price were unmatched by natural gas at that time. The country's natural gas industry is decades behind in comparison. There is a similar situation in China. Although natural gas resources are abundant, with recoverable reserves of not less than 9 trillion cubic meters, it has been restrained by abundant coal resources and coal-based energy production. The natural gas industry is also relatively backward. The proportion of national energy consumption is very small. Since the 1970s, due to the impact of the world economic development by the oil crisis and the increasing environmental problems caused by the use of coal and oil, natural gas liquefaction and storage and transportation technologies have progressed rapidly, making the natural gas market very broad. Not only can it provide power generation, civil industrial and commercial fuels, but it can also replace petroleum as fertilizers and chemical raw materials, and consumption and production have risen sharply. By 2002, the world's natural gas production was nearly 3 trillion cubic meters. It is predicted that around 2020 it will catch up with oil production, reaching 30% of the world's energy structure, and gradually replacing oil, becoming the third peak of energy production and consumption after coal and oil.
| Tips: Link Liquefied Natural Gas The storage and transportation of natural gas at room temperature is not very convenient, but natural gas can be liquefied under low temperature conditions to become liquefied natural gas (LNG). The main component of natural gas is methane (CH4), the critical temperature of its liquefaction is -82.42℃, and higher pressure is required. However, it cannot be liquefied by pressure alone at room temperature. Liquefied natural gas is usually stored in an atmospheric pressure (0.1 MPa) tank at -161°C, and its density is more than 600 times the density of methane under standard conditions. The volumetric energy density of LNG is 72% of gasoline, which is very conducive to transportation, storage and use. When in use, it only needs to heat up and vaporize at the gasification station, enter the pipeline network and transport it to the user, and can also be used as fuel for vehicles, ships, aviation and other vehicles through the filling station. |
However, natural gas is a mineral after all, and it is impossible to regenerate, and no matter how abundant it is, there is a limit to it. Based on the aforementioned proven world resources of 400 trillion cubic meters to 600 trillion cubic meters, mining will be exhausted in about a few decades, and perhaps by the middle of this century, the brilliance of natural gas will be extinguished like oil. Regrettably, the distribution of natural gas resources is also very uneven. Similar to the distribution of oil resources, 70% of natural gas is distributed in the Middle East, Russia and Eastern Europe, and conflicts in competition for energy are inevitable.
I talked about conventional natural gas above, but how should unconventional natural gas be exploited and utilized? Let me talk about coalbed methane first. Coalbed methane is a kind of natural gas that is associated with coal and exists in coal seams in an adsorbed state. The methane content exceeds 95%. It is a high-quality and clean energy. Among all kinds of coal mine safety accidents, most of the gas accidents are caused by coal-bed methane. Methane is also a major "greenhouse gas". If coalbed methane is not recovered and discharged into the environment during coal mining, it will have an important impact on climate warming. Therefore, the extraction and utilization of coal-bed methane is a fundamental measure to prevent coal mine gas explosions and gas emergencies, and it is also a good act to reduce the impact on the environment while obtaining energy. China is a large coal producing country. The amount of methane emitted from coal mining reaches 6 billion cubic meters each year, which is a huge amount. It is not only necessary to recover coal-bed methane from coal mines, but also to use coal-bed methane as a resource by drilling wells for mining. For example, the coal-bed methane production in the United States in 2001 reached 40 billion cubic meters, which is more than China's conventional natural gas production. China's coal-bed methane resources are huge, about 30 trillion cubic meters or more, equivalent to 45 billion tons of standard coal, which is 40% of the recoverable coal reserves, and is a very attractive energy source.
Natural gas hydrate is another unconventional natural gas, a newly discovered energy source. It is a white solid with strong burning ability and looks like ice, so it is commonly called "flammable ice". The core of this hydrate is fuel molecules-most of them are methane, and the outer layer is a framework of water molecules, so it is also called methane hydrate. 1 cubic meter of natural gas hydrate can release 168 cubic meters of methane and 0.8 cubic meters of water, with high energy density. Natural gas hydrate can only exist in a low-temperature and high-pressure environment below -10°C and above 10 MPa (about 100 atmospheres). Once the temperature rises or the pressure drops, methane will escape and the hydrate tends to disintegrate. . The ocean generally has the temperature and pressure conditions of natural gas hydrate, and the ocean continental shelf is the best place for natural gas hydrate formation, usually within the range of 500-1000 meters below the seabed. Gas hydrates also exist in cold permafrost, such as the land in Siberia, Alaska, and the Canadian Arctic Circle.
Natural gas hydrate resources are quite abundant. The total amount of methane contained in the global natural gas hydrate is about 1.8×1016 cubic meters, and its total carbon content is twice the sum of oil, natural gas and coal reserves. Although this kind of natural gas hydrate has brought new energy to mankind, it may also cause serious disasters to the global climate and ecological environment and even the living environment of mankind. The greenhouse effect intensity of methane is dozens of times that of CO2. When such a huge amount of methane in natural gas hydrate is released into the atmosphere, the concentration of methane in the atmosphere will increase thousands of times, and the consequences will be disastrous. However, it is not easy for people to extract this hydrate from the seabed. It can be used as a backup energy source, but when the development technology is not yet mature, don't rush to mine it.















